Atrial fibrillation is a dynamic arrhythmia that requires individual and broad investigations and therapeutic approaches. Comprehensive treatment of atrial fibrillation patients is based on five pillars: 1. prevention of thromboembolic events; 2. symptom control; 3. If possible, restoration or preservation of sinus rhythm; 4. Otherwise good frequency control; 5. treatment of underlying cardiopathy and predisposing factors.
Atrial fibrillation affects 1-2% of the entire population, making it the most common cardiac arrhythmia [1–3]. Due to the increasing prevalence of cardiovascular risk factors and diseases and because of the increasing average age of the general population, we are confronted with a growing number of patients with atrial fibrillation in clinical practice [1–3]. Comprehensive treatment is a challenge. In addition to maintaining quality of life, the risk of thromboembolism and the general increased risk of morbidity and mortality associated with atrial fibrillation must be controlled [1,2]. The findings of epidemiological and clinical research, the advent of new oral anticoagulants (“non-vitamin K antagonist oral anticoagulants” (NOAC), and advances in the field of interventional electrophysiology have revolutionized the treatment of AF. The purpose of this article is to provide an overview of the major concepts and latest paradigms in the treatment of atrial fibrillation.
Initial clarifications and risk stratification
In addition to clinical and laboratory assessment, patients with newly detected electrocardiographically documented atrial fibrillation should also be evaluated by echocardiography and, if necessary, long-term ECG [1,2]. The history and findings obtained form the basis for classifying the type of atrial fibrillation (paroxysmal, persistent, long persistent [>1 year], or permanent), graduating the symptomatology, and assessing the risk of thromboembolism and bleeding. The clarification and primary treatment process is roughly summarized in Figure 1. At this point, it should be noted that the CHA2DS2-VASc score has effectively replaced the CHADS2 score for stroke risk assessment [4].

Thromboembolism prophylaxis in atrial fibrillation
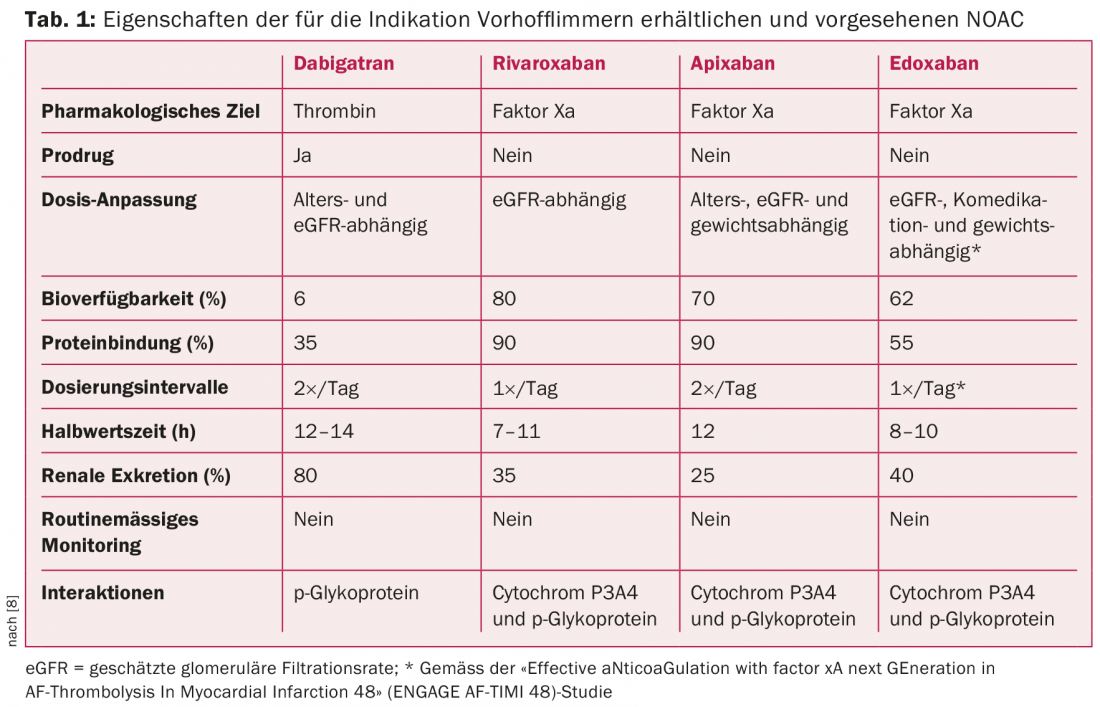
Concurrent with the introduction of the CHA2DS2-VASc score, a paradigm shift in anticoagulation for AF occurred several years ago [1,2,4]. This realignment was largely shaped by the emergence of NOAC [4,5]. To date, three NOACs-apixaban, dabigatran, and rivaroxaban (alphabetical order)-have become established in routine clinical practice for the prophylaxis of thromboembolic events [4–7]. Edoxaban, a fourth drug, is about to be launched (editor’s note: now approved) (Table 1) [7]. NOACs have revolutionized and simplified anticoagulation.
Furthermore, the role of antiplatelet drugs for stroke prophylaxis in atrial fibrillation has been redefined [1,2]. Today, it is generally not recommended to prescribe aspirin for stroke prophylaxis in patients with atrial fibrillation, as this does not provide sufficient thromboembolism prophylaxis and at the same time significantly increases the risk of bleeding [1,4].
Very large randomized trials have shown NOAC to be equivalent or even superior to vitamin K antagonists (VKA) in patients with AF [1,5–8]. In particular, superiority with respect to the risk of intracranial hemorrhage represents an important feature of NOAC [1,5–8]. In general, it should be noted that before initiating anticoagulation therapy, the risk of interaction, bleeding, and general complications should be evaluated, including risk scores (eg, HAS-BLED score) if appropriate. With regard to the increasing prevalence of NOAC, it is important to keep in mind that a reduction in renal function may also consecutively lead to an accumulation of anticoagulants. The extent of accumulation correlates with the severity of renal dysfunction and also depends on the substance used (Tab. 1). One should also be aware of potential drug-drug interactions and that, like VKAs, NOACs may be relevantly affected in their metabolism by co-medication and hepatic function (Tab.1) [4,5].
At times, one is confronted with patients with atrial fibrillation and a high risk of thromboembolism, but who are at risk of bleeding or thromboembolism because of the risk of bleeding or thromboembolism. -complications are not eligible for anticoagulation. It is now known that the left atrial ear is a central source of thrombus formation in atrial fibrillation [9,10]. For well-selected patients who are not eligible for anticoagulation due to contraindications, an alternative has emerged in recent years with the introduction of so-called atrial appendage closure systems to reduce the risk of stroke or stroke-related death. Contain bleeding risk [1,2,9,10]. Several studies have already shown that these atrial ear closure systems can be an effective therapeutic option [9,10].
Rhythm control – Drug options
In addition to thromboembolism prophylaxis, the second central element in atrial fibrillation patients is the therapy of the arrhythmia per se. The treatment strategy should be based on the individual circumstances and is thus guided by the patient’s symptomatology, the duration of the episode of atrial fibrillation, and the overall clinical situation.
Possible strategies for rhythm control in patients with paroxysmal or persistent AF are listed in Figure 2. Symptomatic patients as well as patients with limitation of cardiac function in atrial fibrillation (e.g., tachycardiomyopathy) often benefit from rhythm-controlling therapy [2,3,11]. Depending on the symptoms and duration of the episode of atrial fibrillation, after clarifying the need for anticoagulation resp. after exclusion of intracardiac thrombus, drug or electrical cardioversion should be sought. In general, if AF persisted for more than 48 hours or longer, anticoagulation should be established for at least three weeks before cardioversion and then continued for at least four weeks and indefinitely thereafter depending on the CHA2DS2-VASc score [1,2].
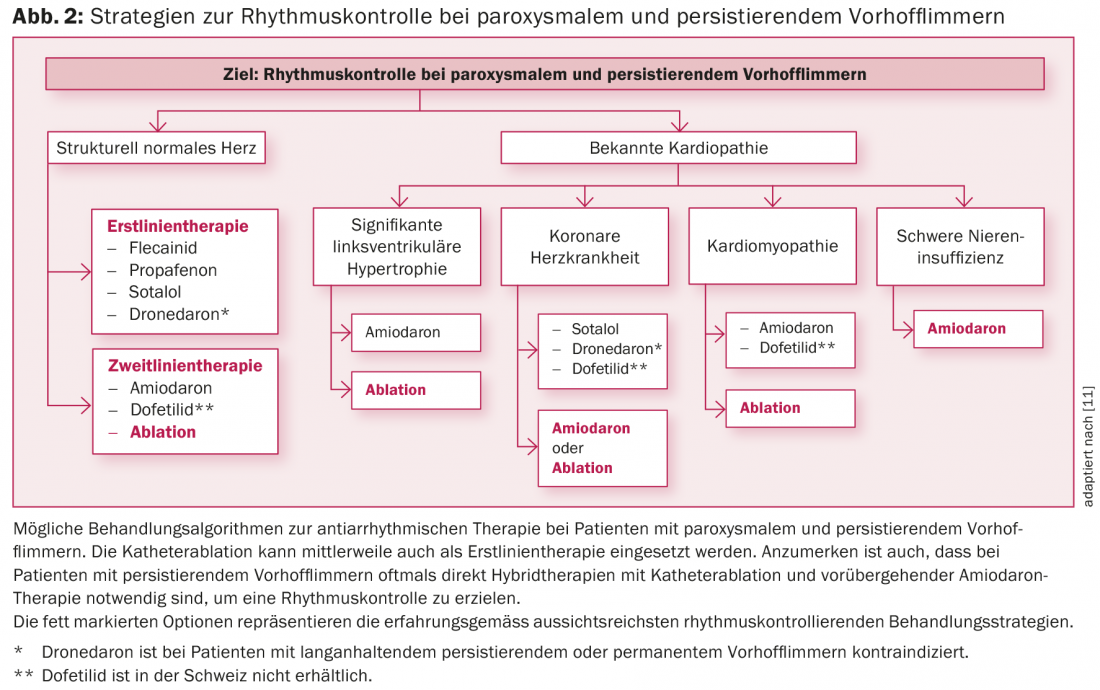
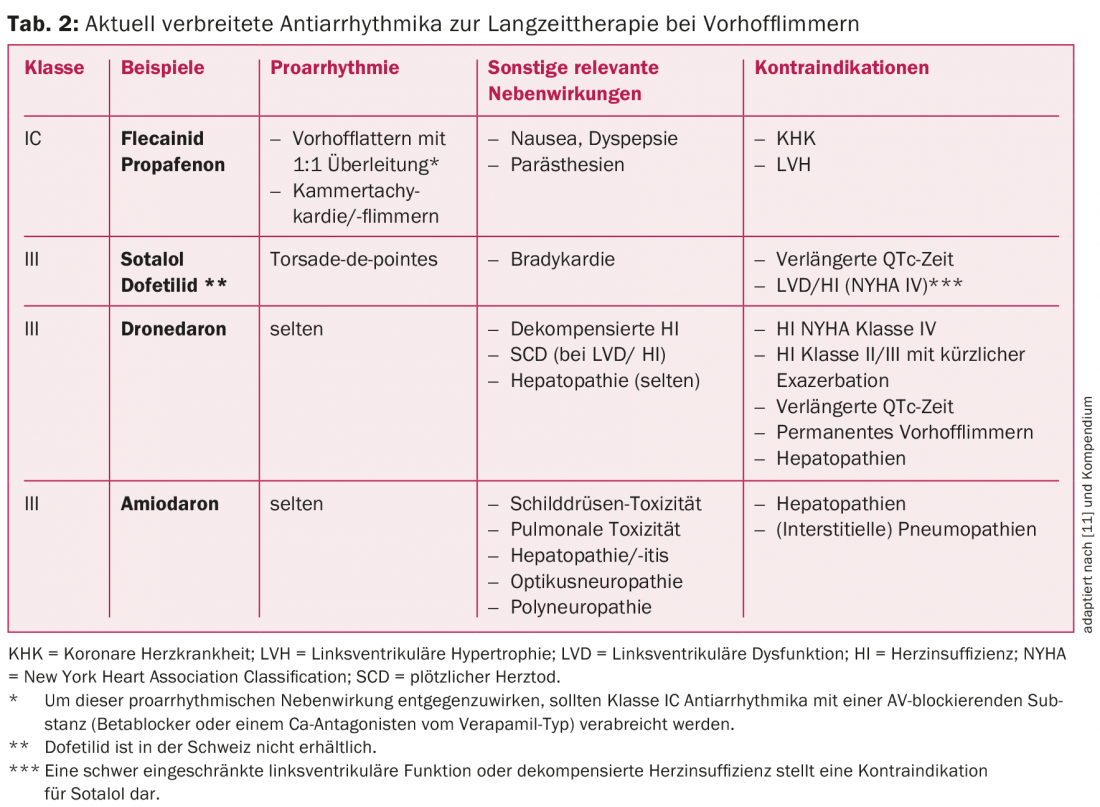
The currently most common antiarrhythmic drugs for long-term rhythm control and their indications in atrial fibrillation are listed in Table 2 and Figure 2, respectively. In patients without structural heart disease, class IC antiarrhythmic drugs (e.g., flecainide or propafenone) in combination with a cardioselective β-blocker are the first choice [1]. Amiodarone and dofetilide (the latter not available in Switzerland) are the therapeutic agents of choice, especially in patients with structural cardiopathy or impaired pump function (fig. 2) [1,3]. Amiodarone is the most potent antiarrhythmic agent available and should be administered on a time-limited basis whenever possible because of its significant side effect and interaction profile [2,3,11]. Newer antiarrhythmic drugs such as vernakalant (only available i.v.) and dronedarone have not been able to establish themselves in the long term due to limited efficacy and unfavorable studies. In particular, dronedarone should be used with caution based on the results of the PALLAS and ANDROMEDA trials [2,3,11]. Thus, in patients with permanent atrial fibrillation resp. Heart failure even contraindicated [2,3,11]. However, because established class IC and III antiarrhythmic drugs also have limited efficacy and AF is a dynamic disease, long-term drug maintenance of rhythm is often not very successful [2,3,11].
Rhythm Control – Electrophysiological Options
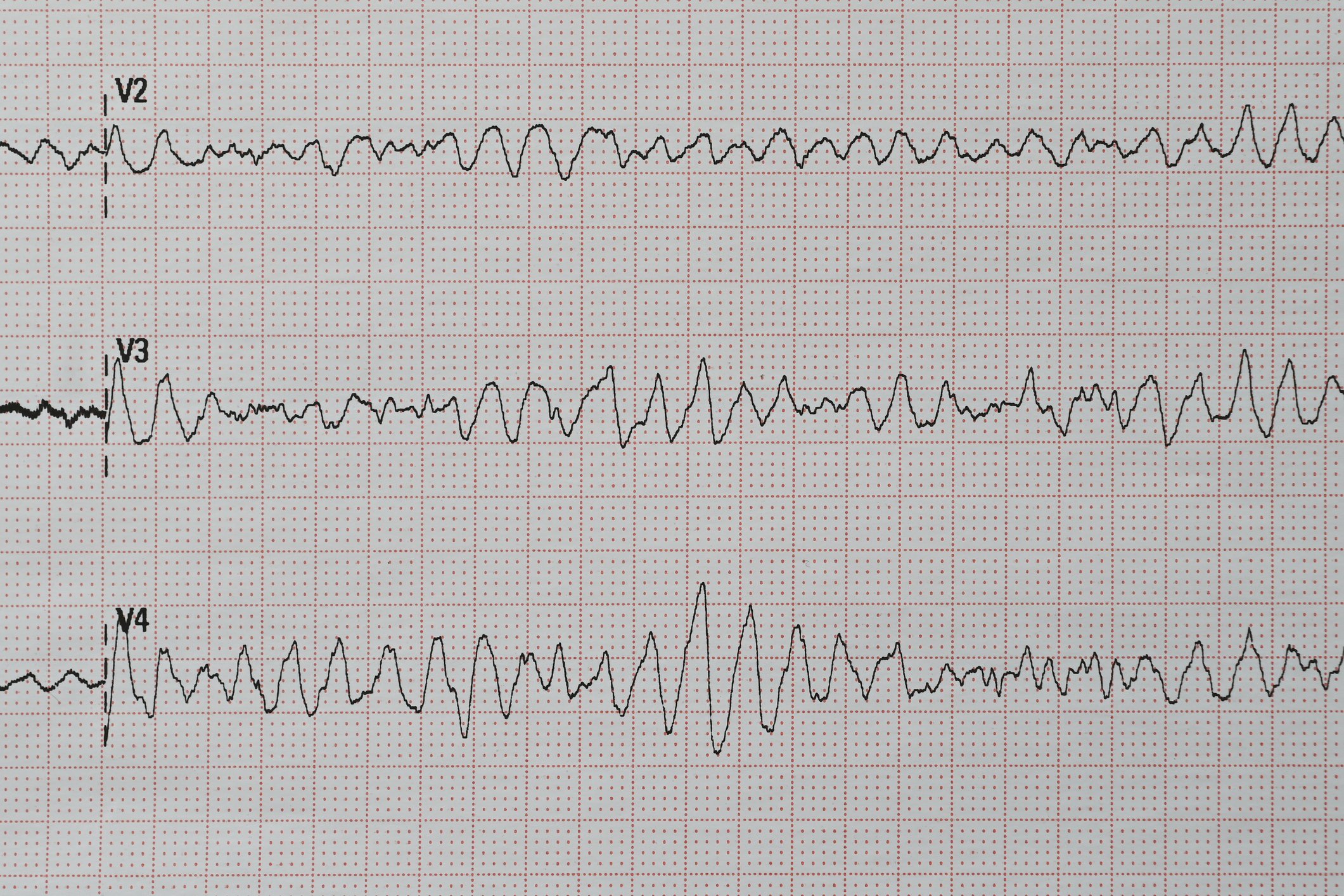
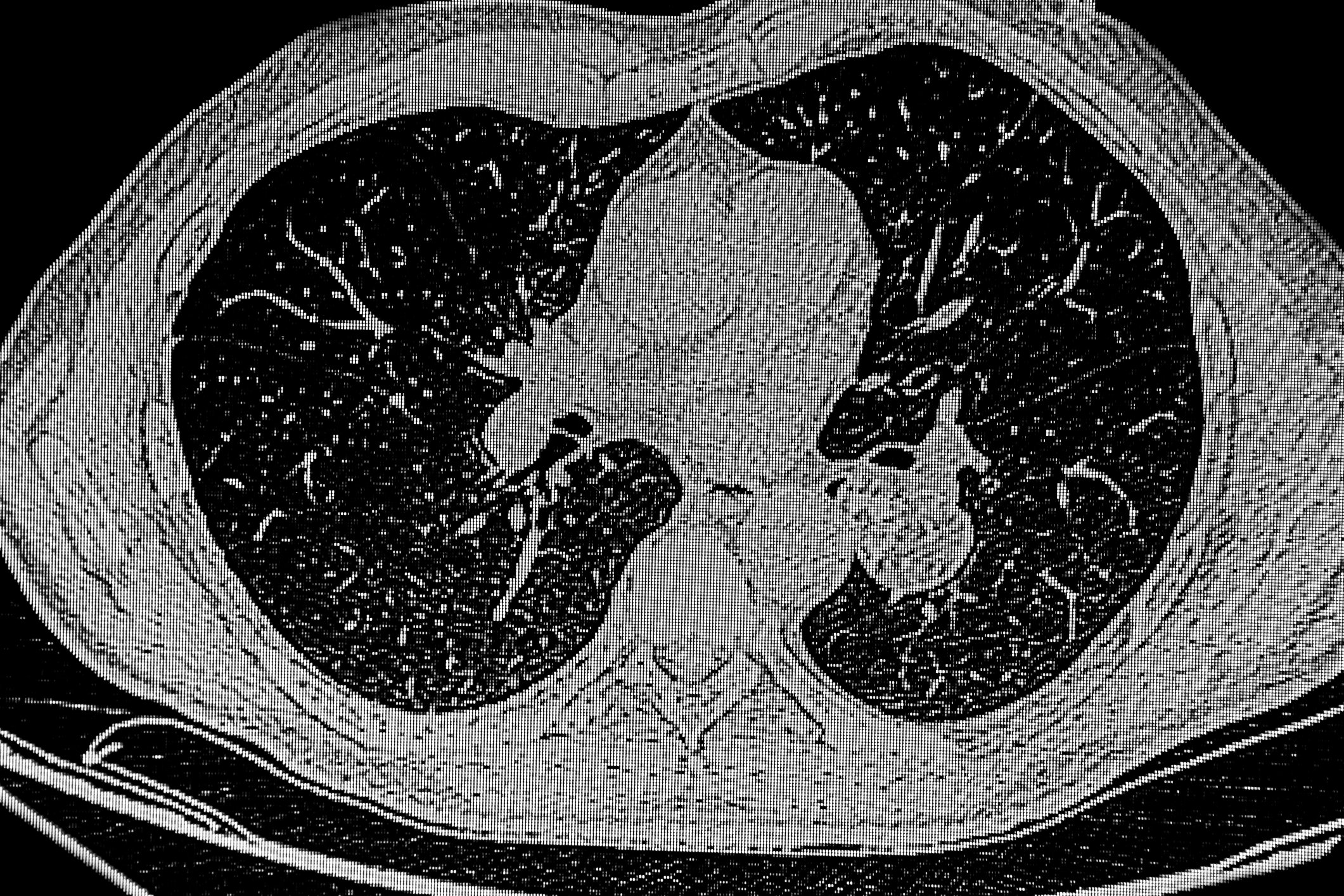
With the growing pathophysiological understanding regarding the development of atrial fibrillation, new therapeutic targets are continuously emerging. Since the performance of the first rhythmosurgical procedures (MAZE procedures) to preserve sinus rhythm, much has happened in the field of interventional AF treatment. Thus, the aim today is to electrically isolate the pulmonary veins in patients with paroxysmal and also persistent atrial fibrillation by means of catheter-based ablation procedures and the placement of sclerotherapy lines, since electrically active foci there are considered important triggers for the arrhythmia (Fig. 3) [1,2,11].
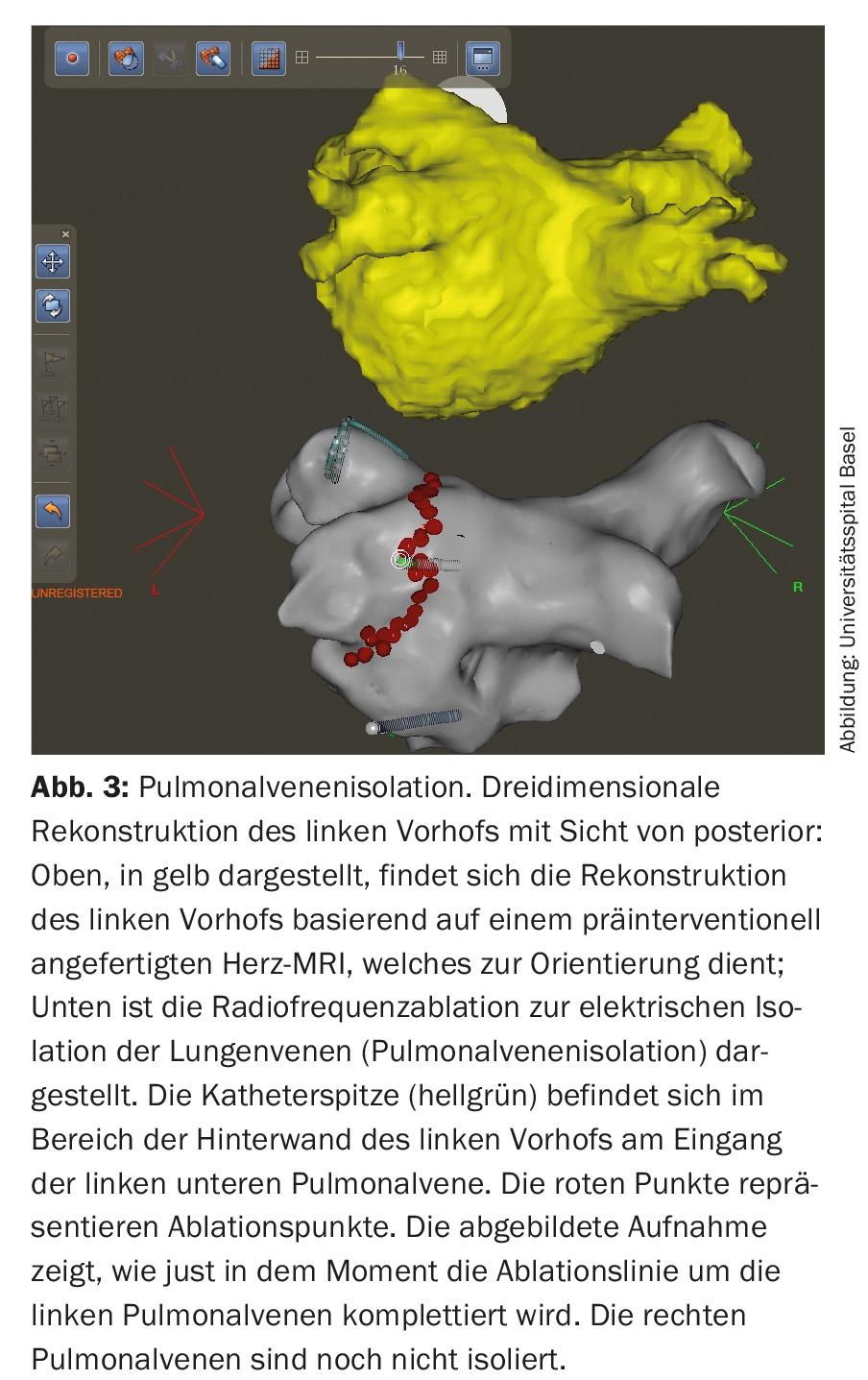
Pulmonary vein isolation is performed by radiofrequency or cryoablation and has become a routine cardiac procedure. The risk of complications is now very low in centers with high numbers of procedures. In addition to complications at the inguinal puncture site (approx. 2%), pericardial tamponade requiring drainage may occur in rare cases (<1%). TIA or stroke also occur rarely (<1%), and the most feared complication, atrioesophageal fistula, occurs very rarely (0.01-0.2%) [2,10,11]. Increasingly, ablation therapy is the primary treatment for many symptomatic patients with AF [1–3,11]. Nevertheless, the indication for interventional therapy should be made only after careful risk-benefit assessment. In general, in addition to patient preference, the following factors must be evaluated: atrial fibrillation type and duration, atrial size, and underlying cardiopathies [1,2,11].
Success rates for atrial fibrillation ablation have improved significantly in recent years. A recently published meta-analysis showed that with catheter ablation, 77% of all patients were free of symptomatic AF after one year, compared with 52% with antiarrhythmic drug therapy [2]. However, experience has shown that approximately one quarter to one third of all patients require a second intervention (so-called redo-ablation) to achieve sustained symptom control [3,11].
Frequency control
In asymptomatic as well as especially elderly and polymorbid patients with atrial fibrillation, the focus is often on heart rate control. This approach is based on the data of several randomized trials (e.g., AFFIRM and RACE trials), which failed to show a discernible mortality benefit of rhythm- versus frequency-controlling therapy in the respective patient populations [2,3,11]. If atrial fibrillation can be accepted because the patient is asymptomatic in this regard, only the ventricular rate is then controlled using a beta-blocker or calcium antagonist [2,3,11]. Digoxin is still used, for example, when frequency control is necessary in the context of decompensated heart failure, but is used by the authors overall only in very selected cases. The recently published TREAT-AF study, a retrospective analysis of more than 120,000 patients, points in the same direction by showing that digoxin is associated with increased mortality in AF [12].
If adequate frequency control is not achieved with medication, interventional frequency control should be sought [2]. This also involves catheter ablation to cut the AV node, which results in control of ventricular rate but also pacemaker dependence [2]. This therapy option is gaining in importance again, as it has a high success rate (>99%) and the positive side effect for the patient that the frequency-blocking drugs (and their side effects) can also be discontinued.
Treatment of risk and concomitant factors
A large number of studies have demonstrated that optimal treatment of classic cardiovascular risk factors (arterial hypertension, diabetes, dyslipidemia, and nicotine consumption) as well as common comorbidities such as obesity, obstructive sleep apnea syndrome, and renal insufficiency has a positive influence on the incidence and course of AF [2,3,11,13].
In a recently published paper, it was impressively shown that rigorous weight reduction in combination with optimal treatment of cardiometabolic risk factors leads to a reduction of symptomatic atrial fibrillation and positively influences cardiac remodeling [13]. The term “upstream therapy” has become fashionable in the context of comprehensive treatment of atrial fibrillation [2,3,11]. This includes drug therapy for factors such as inflammation and fibrosis that promote atrial fibrillation. In this regard, several studies have shown that ACE inhibitors/AT-2 antagonists and statins in particular may have a long-term beneficial effect and counteract atrial fibrillation evolution.
Conflicts of interest
- Matthias Bossard has no conflicts of interest in connection with this article.
- Stefan Osswald: Consulting/lecture fees: Boehringer-Ingelheim
- Michael Kühne: Consulting/lecture fees: Boehringer-Ingelheim, Bayer, Daiichi-Sankyo
Literature:
- Camm AJ, et al: 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012 Nov; 33(21): 2719-2747.
- European Heart Rhythm A, European Association for Cardio-Thoracic S, Camm AJ, et al: Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010 Oct; 31(19): 2369-2429.
- Trulock KM, Narayan SM, Piccini JP: Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014 Aug 19; 64(7): 710-721.
- Lip GY: Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J 2013 Apr; 34(14): 1041-1049.
- Heidbuchel H, et al: EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013 Jul; 34(27): 2094-2106.
- Granger CB, et al: Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011 Sep 15; 365(11): 981-992.
- Giugliano RP, et al: Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013 Nov 28; 369(22): 2093-2104.
- Hart RG, et al: Anticoagulants in atrial fibrillation patients with chronic kidney disease. Nat Rev Nephrol 2012 Oct; 8(10): 569-578.
- Reddy VY, et al: Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014 Nov 19; 312(19): 1988-1998.
- John Camm A, et al: Left atrial appendage closure: a new technique for clinical practice. Heart Rhythm 2014 Mar; 11(3): 514-521.
- Woods CE, Olgin J: Atrial fibrillation therapy now and in the future: drugs, biologicals, and ablation. Circ Res 2014 Apr 25; 114(9): 1532-1546.
- Turakhia MP, et al: Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol 2014 Aug 19; 64(7): 660-668.
- Abed HS, et al: Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013 Nov 20; 310(19): 2050-2060.
CARDIOVASC 2015