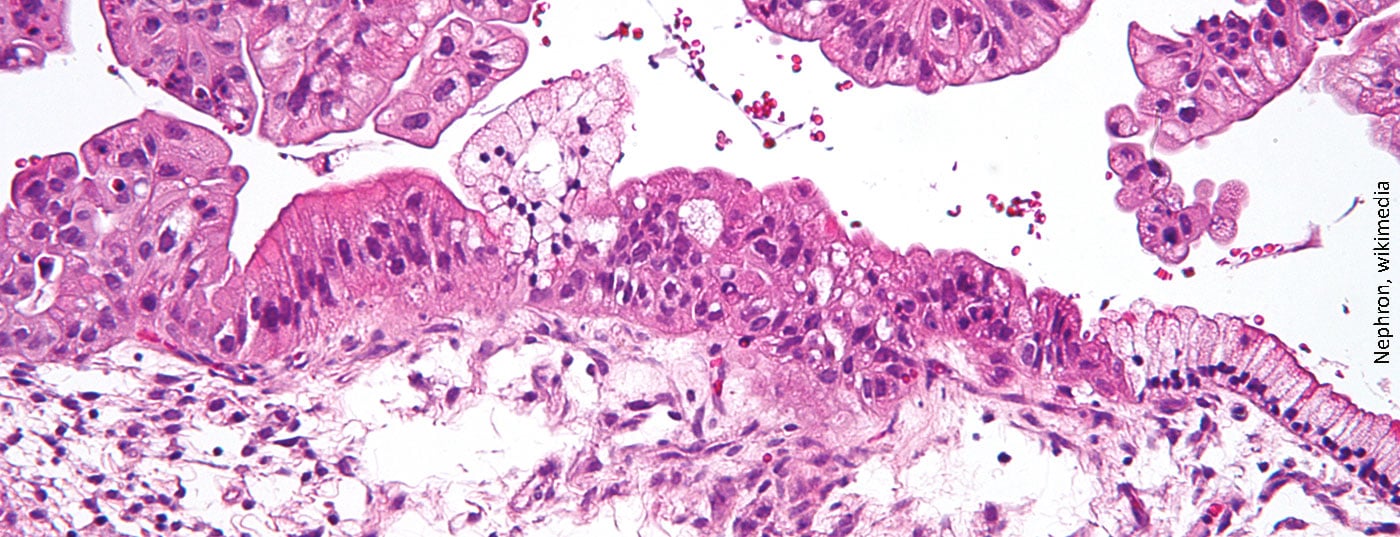
More women die from ovarian cancer than from any other tumor – mainly because it is usually detected very late. PARP inhibitors have been developed for the treatment of recurrent ovarian cancer and have been shown to increase progression-free survival (PFS) as maintenance therapy.
Every year, approximately 580 women are diagnosed with ovarian cancer in Switzerland. Almost 75% of patients do not survive this disease. One reason is that in about three quarters of those affected, the tumor is detected at a very late stage due to the non-specific symptoms. Due to the aggressive course of the cancer, this is associated with a very poor prognosis. About 80% of patients relapse within three years after surgery and chemotherapy.
Early detection options are lacking
The risk of developing ovarian cancer is influenced by age, obesity, childlessness, early menarche, late menopause, and family history. It is also significantly increased if there is a mutation in the BRCA genes. Then the lifetime prevalence increases twentyfold. Currently, there is a lack of reliable screening methods to identify the disease in time. At the time of diagnosis, the cancer has often already spread to nearby organs. However, the later the diagnosis is made, the more limited the treatment options. If surgery, cytostatics and angiogenesis inhibitors do not work and recurrence occurs, PARP inhibitors can now be used.
These interfere with the DNA repair mechanism and lead to tumor apoptosis by means of genomic instability. Tumor cells use PARP enzymes to repair their DNA damage. This also includes damage caused by cytostatic drugs. Single-strand breaks can no longer be repaired by PARP inhibitors. The double-strand breaks that occur during the next cell division then lead to cell death in cells with impaired DNA double-strand repair.
Maintenance therapy convinces
A randomized, double-blind, placebo-controlled phase III study evaluated the PARP inhibitor niraparib versus placebo in the treatment of 733 patients with stage III or IV ovarian cancer after platinum-based chemotherapy. Currently, the drug is approved in Switzerland as maintenance therapy for platinum-sensitive recurrent ovarian cancer after response to platinum-based chemotherapy, regardless of BRCA mutation status, subject to limitatio. The efficacy of PARP inhibitor as maintenance therapy, as measured by PFS, in patients with homologous recombination deficient tumors and in patients in the overall population, as determined by hierarchical testing, was evaluated.
Results of a Phase III study demonstrate efficacy
A total of 373 patients (50.9%) with tumors with homologous recombination deficiency were included. Among patients in this category, median progression-free survival was significantly longer in the niraparib group than in the placebo group (21.9 months vs. 10.4 months; p<0.001). In the overall population, the corresponding PFS was 13.8 months and 8.2 months (HR, 0.62; 95% CI, 0.50 to 0.76; p<0.001) (Fig. 1). In a 24-month interim analysis, overall survival was 84% in the verum group and 77% in the placebo group (HR, 0.70; 95% CI, 0.44 to 1.11). The most common grade 3 or higher adverse events were anemia (in 31.0% of patients), thrombocytopenia (in 28.7%), and neutropenia (in 12.8%).
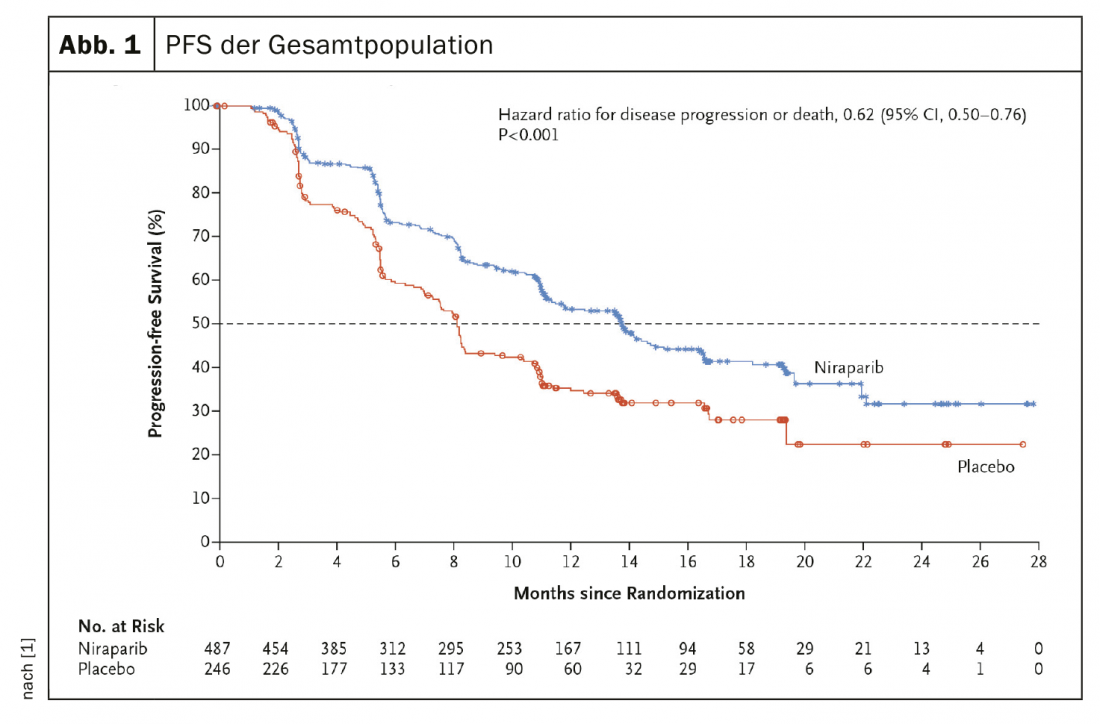
PARP inhibitor leads to significantly improved PFS
The experts summarized that among patients with newly diagnosed advanced ovarian cancer who responded to platinum-based chemotherapy, those who received the PARP inhibitor had significantly longer progression-free survival than those who received placebo. This result was independent of homologous recombination deficiency.
Further reading:
- González-Martín A, et al: Niraparib in atients with Newly Diagnosed Advanced Ovarian Cancer, NEJM 2019; Online first. DOI: 10.1056/NEJMoa1910962.
Source: European Society for medical Oncology (ESMO) 2019
InFo ONCOLOGY & HEMATOLOGY 2019; 7(5): 28 (published 10/17/19, ahead of print).