Dietary changes and exercise often prove insufficient as measures against morbid obesity. Until now, bariatric surgery has been the only goal-oriented measure for many obese patients. This has changed in the meantime. With incretin mimetics, substantial weight loss can be achieved with parallel improvement in quality of life. While GLP-1-RA mimic the incretin hormone glucagon-like peptide(GLP)-1, dual GIP/GLP-1 agonists also activate glucose-dependent insulinotropic peptide (GIP) receptors.
Obesity is a complex, multifactorial disease that increases the risk for secondary diseases such as type 2 diabetes, cardiovascular disease, kidney damage, and cancer [1–3]. Both obesity and diabetes are caused and influenced by a variety of genetic determinants and environmental factors [4]. Obesity occurs as a result of a positive energy balance – however, the causes for the imbalance between supplied and consumed energy are heterogeneous and complex.
Incretin analogues as “game changers” – losing weight without starving yourself
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) are incretins. These substances mimic endogenous hormones of the gastrointestinal tract that make you feel full, increase fat burning and stimulate insulin secretion [5,6]. “Obesity drugs can replicate one, two, or three incretins in a molecule and thus have different potencies,” explained Prof. Susanne Reger-Tan, MD, Director of the Diabetes Center of Excellence at the Department of Endocrinology, Diabetology, and Metabolism at Essen University Hospital (D) [1]. Treatment with incretin analogues, often referred to in the media as “weight loss injections,” leads to substantial weight loss in people with obesity, and without the nagging feeling of hunger. “Based on current knowledge, this new drug approach is truly a gamechanger in the treatment of obesity,” said Prof. Reger-Tan. Nevertheless, it is an intervention in the organism. “Use should be after careful risk-benefit analysis within the indications for which these agents are approved, and should be accompanied by baseline multimodal therapy to achieve sustained results.”
Two GLP-1 RAs and one dual GIP/GLP-1 agonist are approved in this country
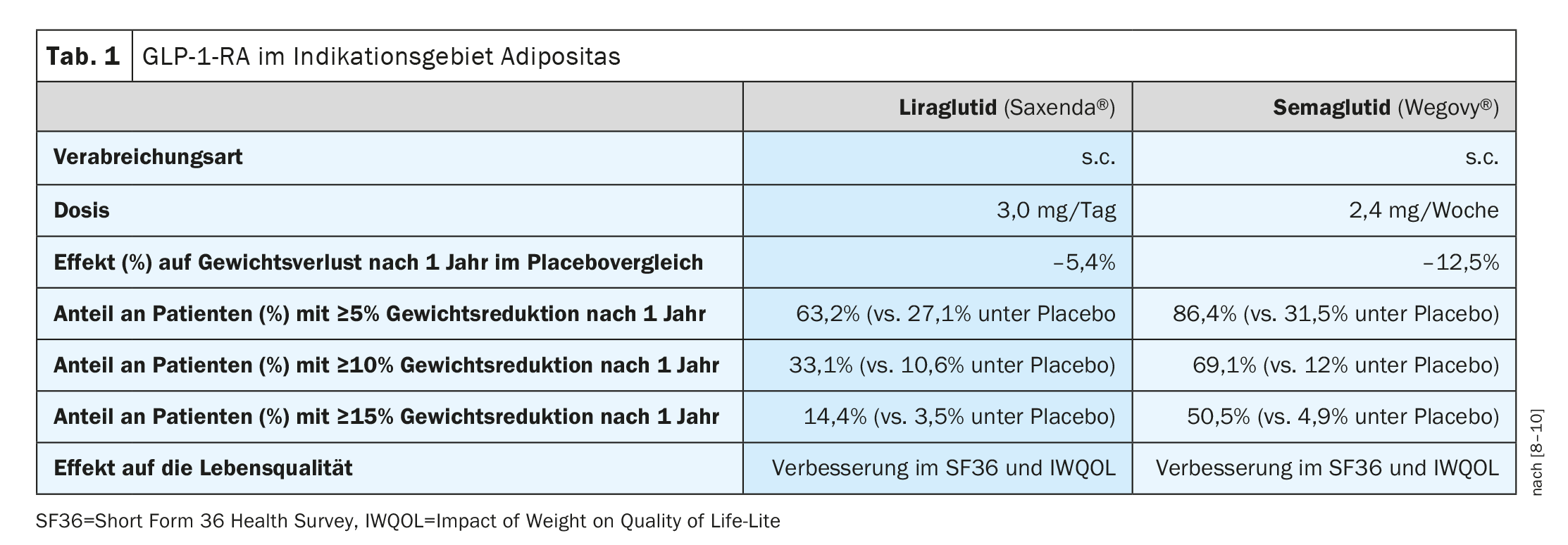
In addition to Saxenda® (liraglutide 3 mg), which has been available in Switzerland for some time for obesity therapy in obese patients aged 12 years and older, Wegovy® (semaglutide 2.4 mg) also received Swissmedic approval for this indication in 2022 (Table 1) [7]. Both active ingredients belong to the group of GLP-1 receptor agonists (GLP-1-RA) – also known as “incretinimetics/ incretin analogues”. Both Saxenda® and Wegovy® are subcutaneously administered long-acting analogues of the incretin GLP-1. The effects of these substances are based, among other things, on promoting insulin synthesis and release and inhibiting the release of glucagon. Gastric emptying is delayed, which increases the feeling of satiety. While Saxenda® is an approved drug for adults with obesity, Wegovy® is not covered by health insurance in Switzerland to date. A temporary limitation for Saxenda® [13] applies until 30.06.2026 . In addition to a BMI ≥35 kg/m2 or ≥28 kg/m2 **, it is required that one eats at least 500 kcal less daily than the body burns energy (“500 kcal/day deficit diet”). In addition, patients must demonstrate an increase in physical activity (e.g., pedometer). Pretreatment with GLP-1-RA and previous or planned bariatric surgery are among the exclusion criteria. Prescription may be made exclusively by specialists in endocrinology/diabetology and at obesity centers. According to the current limitation [13] Saxenda® must not be combined with other GLP-1-RAs, gliptins, SGLT-2 inhibitors or insulin. When Saxenda® is used for the first time, a weight reduction of at least 5% or 7% of the initial body weight should be achieved after 16 weeks of treatment.
** or BMI ≥28 kg/m2 if additional weight-related comorbidities such as prediabetes or type 2 diabetes mellitus, arterial hypertension, or dyslipidemia are present.
| Dual GIP/GLP-1 agonist Tirzepatide (Mounjaro®) is a once-weekly agonist of the GIP and GLP-1 receptors. It is a single molecule that activates the receptors for glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), both of which are natural incretin hormones. Both GIP and GLP-1 receptors are found in areas of the human brain relevant to appetite regulation. Tirzepatide has been shown to decrease food intake and modulate lipid metabolism. This resulted in significant weight loss in studies of obese patients. Research is also underway on triagonists that address the glucagon receptor in addition to GIP and GLP-1 receptors. |
| to [12,13] |
Tirzepatide (Mounjaro®) (box ), a dual GIP/GLP-1 agonist, was also approved in 2022. Tirzepatide activates both glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) receptors. The starting dose of tirzepatide is 2.5 mg once weekly. After four weeks, the dose is increased to 5 mg once a week. If necessary, the dose may be increased in increments of 2.5 mg, after at least four weeks at the current dose. The maximum dose is 15 mg once a week. In the SURPASS trial program, depending on the dose (5, 10, or 15 mg), tirzepatide achieved a 7-14.5% reduction in body weight within 40 to 52 weeks of treatment [11].
Literature:
- “Obesity: new era in weight loss therapy by weight loss injections,” German Society of Endocrinology (DGE), Sept. 05, 2023.
- Collaborators GBDO, et al: Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017; 377: 13-27.
- Pearson-Stuttard J, et al: Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2018; 6:e6-e15.
- “Immunometabolism and Gut Microbiome Project ” www.udem.insel.ch/de/lehre-und-forschung/
research-focus/adipositas/immunometabolism-and-gut-microbiome-project (last accessed 09.10.2023) - Nogueiras R, Nauck MA, Tschop MH: Gut hormone co-agonists for the treatment of obesity: from bench to bedside. Nat Metab 2023; 5: 933-944.
- Ussher JR, Drucker DJ: Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol 2023; 20: 463-474.
- Drug Information, www.swissmedicinfo.ch (last accessed Oct. 09, 2023).
- Wilding JPH, et al: Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021; 384(11): 989-1002.
- Pi-Sunyer X, et al: A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med 2015; 373(1): 11-22.
- Kolotkin RL, et al: Improvements in health-related quality of life over 3 years with liraglutide 3.0 mg compared with placebo in participants with overweight or obesity. Clin 2018; 8(1): 1-10.
- Müller TD, Blüher M: Obesity therapy – will pharmacotherapies be the alternative to metabolic surgery? [Obesity treatment: will pharmacotherapies replace metabolic surgery in the future?]. Inn Med (Heidelb) 2023; 64(7): 629-635.
- “Mechanism of action of tirzepatide deciphered,” www.helmholtz-munich.de/newsroom/news/artikel/unraveling-the-mode-of-action-of-tirzepatide (last accessed Oct. 10, 2023).
HAUSARZT PRAXIS 2023; 18(10): 36-37