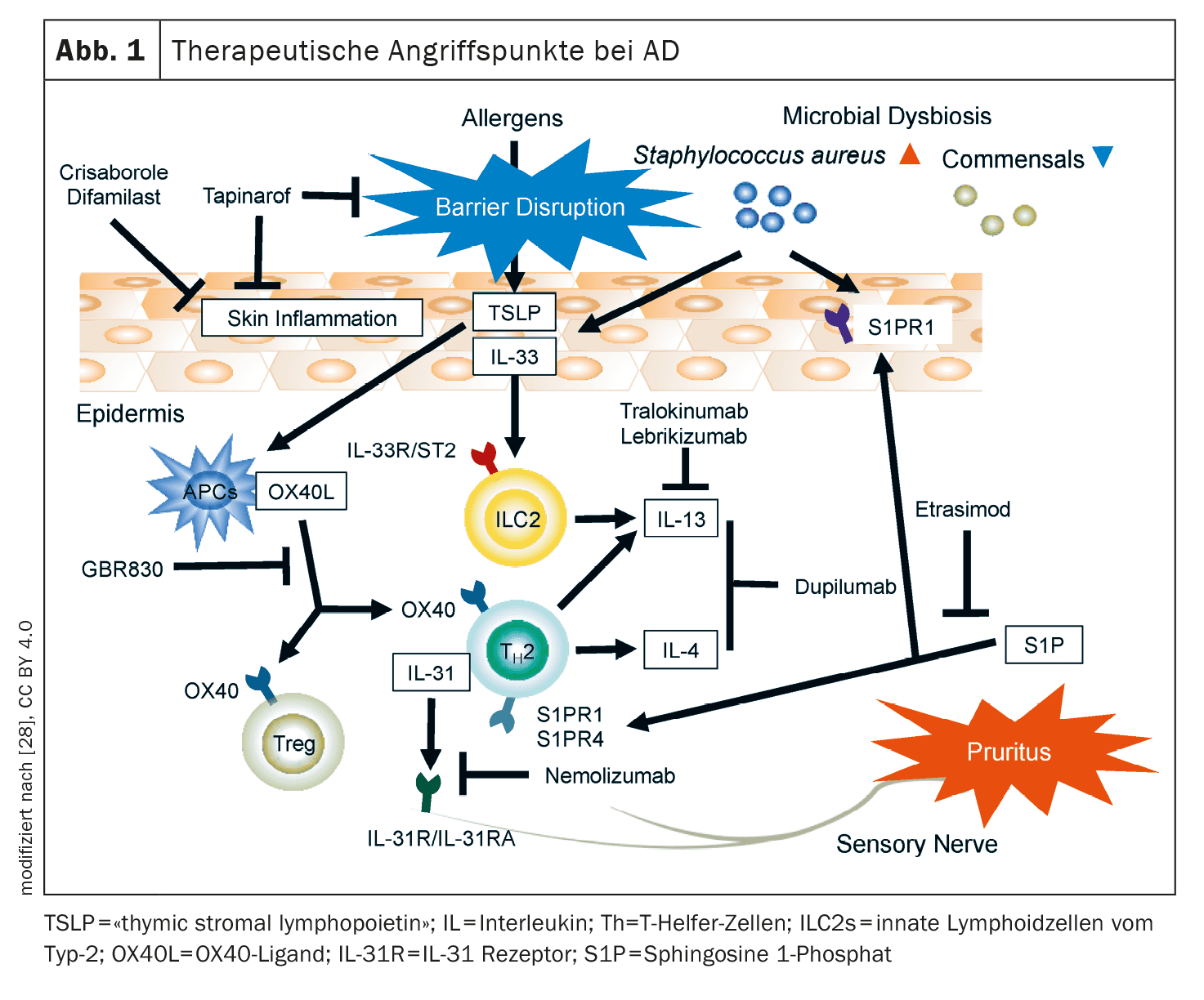
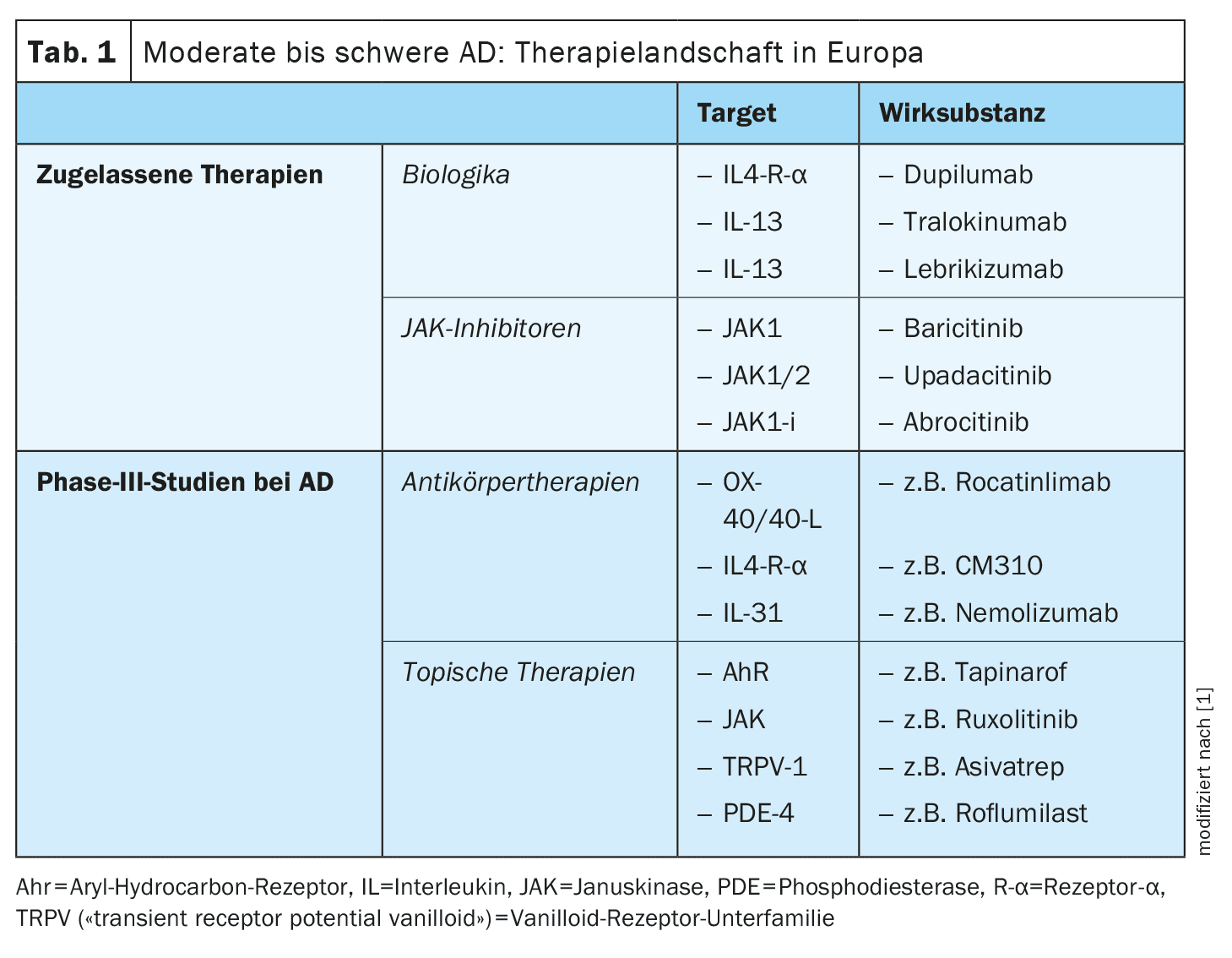
The arsenal of currently researched active substances in the indication area of atopic dermatitis (AD) is considerable. In addition to anti-OX40/OX40L antibodies, CM310, nemolizumab and neurokinin receptor 1 antagonists, there are also several topical anti-inflammatory therapies, such as tapinarof, ruxolitinib, delgocitinib, roflumilast, difamilast and asivatrep. Some of these innovative therapies have already been approved in some countries.
The treatment options for atopic dermatitis (AD) have expanded significantly in recent years, so that a wide range of effective and safe topical and systemic therapies are currently available. The demands and expectations of therapy have increased: the aim is not only to achieve an optimal risk-benefit profile, but also to achieve treatment effects that are as long-lasting as possible. However, the “unmet needs” also include certain subgroups of patients who do not respond adequately to the currently available treatment options or do not tolerate them. The convenience factor should not be neglected either; the treatment should be as easy as possible to integrate into the patient’s everyday life and help to achieve a rapid and lasting improvement in quality of life. With the development of new active ingredients, targets and dosage forms, attempts are being made to close these treatment gaps [1–3].
New immunomodulatory antibody therapies
Among the antibody therapies, the OX40/OX40L axis, IL-31 and the neurokinin-1 receptor on sensory neurons are promising targets in AD [1,2]. In addition, new representatives of established target structures, such as the IL-4 receptor-α (IL4R-α), are being researched.
OX40/OX40L axis: OX40 is expressed on T cells in lesional AD skin [4]. Binding of the OX40 ligand to OX40 leads to T2-driven immune differentiation, which is targeted by various new drugs [5].
- Rocatinlimab (AMG451/KHK4083) is a subcutaneously administered anti-OX40L antibody that is currently being investigated in Phase III trials in adults with moderate to severe AD. In a Phase IIb study, the dosage of 300 mg every 2 weeks (q2w) proved to be the most effective with an EASI reduction of 61% at week 16 [3]. The most common adverse events ( AEs), which occurred more frequently than with placebo, were pyrexia (17%), nasopharyngitis (14%) and chills (11%) [6]. Proteomic analyses showed that rocatinlimab reduced Th2/Th22- and pruritus-associated mediators at week 16, accompanied by a downregulation of Th2, Th1/17 and Th22-associated genes by week 52.
- Other OX-40 inhibitors: currently in the pipeline for AD are GBR830 (anti-OX40), telazorlimab (ISB830) and amlitelimab (KY1005; anti-OX40L) [7].
IL-4Rα: In addition to the already approved IgG4 antibody dupilumab, which binds to IL-4Rα and thus inhibits the signaling pathway of IL-4 and IL-13, further active substances are currently in development.
- CBP-201: This is an IL-4Rα inhibitor that is currently being investigated in two Phase II studies (NCT04444752, NCT05017480) and has achieved promising results [8]. A mean change in EASI scores of -63.0% (every 2 weeks, q2w) and -65.4% (every 4 weeks, q4w) was achieved with CBP-201, compared to -40.7% in the placebo group [8].
- CM310: This anti-IL-4Rα antibody is currently being investigated in phase II (NCT04805411) and phase III studies (NCT05265923, NCT04893707) [8].
IL-31: This cytokine has been shown to be exceedingly significant in itch pathogenesis and serum IL-31 levels correlate with AD severity [9]. IL-31 is involved in the epidermal barrier disruption typical of AD and activates pruriceptive neurons, which release neuropeptides that drive local inflammation in the skin by attracting Th2 cells.
The IL-31 receptor α chain (IL-31Rα) is an important therapeutic target that is antagonized by nemolizumab [10,11]. In the USA, this biologic is approved for the treatment of prurigo nodularis and in Japan also for AD-associated itching in patients aged ≥13 years [10,12].
- Nemolizumab: With nemolizumab 60 mg (q4w) as an add-on to standard topical therapy, 66% achieved a reduction in pruritus and 78% an EASI reduction at week 68 [10]. The most common AEs were nasopharyngitis (33.9%) and AD (25.2%) [11]. Phase III trials of nemolizumab and Phase II trials in children (2-11 years) with moderate to severe AD are currently ongoing.
Substance P (SP) and the neurokinin-1 receptor (Nκ1R): It is known that these tachykinins are involved in the peripheral and central transmission of histamine-independent itch and that AD patients have elevated SP levels in lesional skin and serum [13,14]. Nκ1R are mainly located in the dorsal root ganglion and dorsal horn of the spinal cord.
- Whether Nκ1R antagonists are effective in AD is still not entirely clear, especially since aprepitant with concomitant standard topical therapy did not lead to a significant improvement in pruritus in AD in a placebo comparison. In contrast, serlopitant significantly reduced chronic pruritus in PN [13,15]. Phase III study data on serlopitant and tradipitant with regard to pruritus in AD have not yet been published [14].
New topical substances for anti-inflammatory therapy
In addition to subcutaneously and orally administered systemic therapeutics, topical anti-inflammatory therapies also play an important role, particularly for AD patients with a limited body surface area (BSA) of <10%. Currently at an advanced stage of development are agents that target JAK receptors and TRPV (“transient receptor potential vanilloid”) , as well as agonists of the aryl hydrocarbon receptor (AhR) [16].
Topical JAK inhibitors: Compared to the systemic application of JAK-i, topical application carries significantly fewer side effect risks.
- Ruxolitinib: Ruxolitinib cream inhibits JAK1/2 and was approved by the US Food and Drug Administration (FDA) in 2021 for the treatment of mild to moderate AD in ≥12-year-olds [17]. In the approval-relevant study, good efficacy was also achieved with regard to itching and tolerability also proved to be good. Nasopharyngitis was reported as the most common AE [17].
- Delgocitinib: This topical JAK-i blocks all JAK molecules and was approved for the treatment of AD in Japan in 2020 [18]. In a phase III study, a four-week treatment with 0.5% delgocitinib cream led to an improvement in local inflammation of around 45%. The most common side effects were folliculitis (2.4%), acne (2.2%) or irritation (1.8%) at the site of application [19].
- Other topical JAK-i are currently being investigated in clinical trials: Brepocitinib (IIb), ATI-1777, Jaktinib and SHR0302 (Phase II/III) [20].
Topical AhR: Aryl hydrocarbon receptors (AhR) are expressed in all skin cells, including keratinocytes and dendritic cells, can be activated by numerous exogenous and endogenous metabolites and mediate epidermal differentiation [22].
- Tapinarof cream 1%: This is an AhR agonist that was approved by the FDA in 2022 for the treatment of plaque psoriasis [23]. Tapinarof modulates gene expression by activating AhR signaling pathways, resulting in a downregulation of type 2 inflammation (IL-4, IL-13), normalizing the skin barrier and contributing to a reduction in oxidative stress. In a 12-week, double-blind, randomized Phase II study, adolescent and adult AD patients using 0.5% or 1% tapinarof cream vs. vehicle preparation showed good improvement of eczematous lesions and pruritus [24]. AEs reported were nasopharyngitis, upper respiratory tract infections, worsening of AD and folliculitis [24]. Phase III studies on tapinarof are currently underway [22].
Topical phosphodiesterase-4 (PDE4)-i: crisaborole, difamilast and roflumilast inhibit the release of certain cytokines involved in the inflammatory process and have been shown to improve the skin’s barrier function [25–27]. Inhibition of PDE-4 results in an increase in cAMP, which leads to a downregulation of NFκB, an important modulator of cytokine production (e.g. IL-4, IL-5, IL-10).
- Crisaborole: This topical PDE-4 inhibitor has been approved in the USA and the EU, but was previously only available on the European market to a limited extent.
- Roflumilast and difamilast: These representatives of the topical PDE-4 inhibitors are currently being researched and may soon be available for the treatment of mild to moderate AD [17,22].
Topical TRPV-1 antagonists: TRPV (“transient receptor potential vanilloid“)-1, i.e. a subfamily of vanilloid receptors, are found on keratinocytes, dendritic cells and sensory neurons and are overexpressed in lesional AD skin. TRPV-1 modulates inflammatory processes as well as histamine-dependent and -independent itching by triggering the release of central neuropeptides such as substance P and CGRP (calcitonin gene-related peptide).
- Asivatrep: This selective TRPV-1 antagonist led to improvements in eczematous lesions and pruritus in a randomized vehicle-controlled phase III study in ≥12-year-old patients with mild to moderate AD [21].
Literature:
- Müller S, Maintz L, Bieber T: Treatment of atopic dermatitis: Recently approved drugs and advanced clinical development programs. Allergy 2024; 79(6): 1501–1515.
- Buhl T, Werfel T: [Atopische Dermatitis – Perspektiven und unerfüllte medizinische Bedarfe]. JDDG 2023; 21(4): 349–354.
- Lé AM, Torres T: OX40-OX40L Inhibition for the Treatment of Atopic Dermatitis-Focus on Rocatinlimab and Amlitelimab. Pharmaceutics 2022 Dec 8; 14(12): 2753.
- Nakagawa H, et al.: Safety, tolerability and efficacy of repeated intravenous infusions of KHK4083, a fully human anti-OX40 monoclonal antibody, in Japanese patients with moderate to severe atopic dermatitis. J Dermatol Sci 2020; 99: 82–89.
- Elsner JS, et al.: The OX40 Axis is associated with both systemic and local involvement in atopic dermatitis. Acta Derm Venereol 2020; 100: adv00099.
- Guttman-Yassky E, et al.: An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study. Lancet 2022; 401(10372): 204–214.
- Clinicaltrials, https://clinicaltrials.gov, status 2022.
- Facheris P, et al.: The translational revolution in atopic dermatitis: the paradigm shift from pathogenesis to treatment. Cell Mol Immunol 2023; 20(5): 448–474.
- Datsi A, et al.: Interleukin-31: the «itchy» cytokine in inflammation and therapy. Allergy 2021; 76: 2982–2997.
- Kabashima K, et al.: Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: results from two phase III, long-term studies. Br J Dermatol 2022; 186: 642–651.
- Kabashima K, et al.: Trial of Nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med 2020; 383: 141–150.
- Keam SJ: Nemolizumab: First Approval. Drugs. 2022; 82: 1143–1150.
- Ständer S, et al.: Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. JAAD 2019; 80: 1395–1402.
- Welsh SE, et al.: Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: results from EPIONE, a randomized clinical trial. JEADV 2021; 35: e338–e340.
- Yosipovitch G, et al.: Serlopitant for the treatment of chronic pruritus: results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. JAAD 2018; 78: 882–891.e10.
- Bieber T: Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov 2022; 21: 21–40.
- Kleinman E, et al.: What’s new in topicals for atopic dermatitis. Am J Clin Dermatol 2022; 23: 595–603.
- Chovatiya R, Paller AS: JAK inhibitors in the treatment of atopic dermatitis. JACI 2021; 148: 927–940.
- Nakagawa H, et al.: Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. JAAD 2020; 82(4): 823–831.
- Bieber T: Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov 2022; 21: 21–40.
- Park CW, et al.: Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). JACI 2022; 149: 1340–1347.e4.
- Freitas E, Gooderham M, Torres T: New topical therapies in development for atopic dermatitis. Drugs 2022; 82: 843–853.
- Keam SJ: Tapinarof cream 1%: first approval. Drugs 2022; 82: 1221–1228.
- Paller AS, et al.: Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. JAAD 2021; 84: 632–638.
- Zebda R, Paller AS. Phosphodiesterase 4 inhibitors. JAAD 2018; 78(3 Suppl 1): 43–S52,
doi: 10.1016/j.jaad.2017.11.056. - Schlessinger J, et al: Safety, effectiveness, and pharmacokinetics of Crisaborole in infants aged 3 to <24 months with mild-to-moderate atopic dermatitis: A phase IV open-label study (CrisADe CARE 1); Am J Clin Dermatol 2020; 21(2): 275–284.
- Saeki H, et al.: Difamilast ointment in adult patients with atopic dermatitis: a phase 3 randomized, double-blind, vehicle-controlled trial. JAAD 2022; 86(3): 607–614, doi: 10.1016/j.jaad.2021.10.027
- Yamamura K, Nakahara T: The Dawn of a New Era in Atopic Dermatitis Treatment. Journal of Clinical Medicine 2022; 11(20): 6145. www.mdpi.com/2077-0383/11/20/6145, (last accessed 04.12.2024).
DERMATOLOGIE PRAXIS 2024; 34(6): 22–24