Long-term anti-inflammatory treatment options are needed for sustainable management of chronic recurrent atopic eczema. Apart from topically applicable steroids and calcineurin inhibitors, new drug candidates for topical therapy are in advanced stages of clinical development or have already cleared the FDA and/or EMA approval hurdles. These include representatives of the PDE-4 inhibitors, the JAK inhibitors, and the aryl hydrocarbon receptor agonists.
Every three years, the Skin Inflammation & Psoriasis International Network (SPIN) congress gathers about 1500 participants from about 90 countries to exchange information about current developments in the field of inflammatory dermatoses [1]. It is an important international meeting focusing on patient management and therapeutic strategies. Experts from around the world will share their experiences and provide insight into the latest results and clinical studies. Dr. Razvigor Darlenski, MD, Department of Dermatology and Venereology, Acibadem Cityclinic Tokuda Hospital, Sofia (Bulgaria) reported on innovations in topical treatment of atopic dermatitis [2].
TCI: Tacrolimus and pimecrolimus are effective and safe – even in children
Topical corticosteroids (TCS) have long been considered the first-line therapy, but lack of adherence has been shown to be a problem in many affected individuals due to side effect risks (e.g., skin atrophy) [3]. The topical calcineurin inhibitors (TCI) tacrolimus ointment 0.03% and 0.1% (Protopic®) and pimecrolimus cream 1% (Elidel®) are safe and effective alternatives to topical corticosteroids [4]. TCIs act specifically on T cell-mediated inflammation and therefore have no steroid-typical side effects, such as atrophy of the skin. In contrast to systemic therapy with calcineurin inhibitors, large prospective studies were able to refute an increased cancer risk of topically applied calcineurin inhibitors, one of which was published in the Journal of the American Academy of Dermatology in 2020 (box).

PDE-4 inhibitors: Crisaborol already approved in some countries
Crisaborole ointment 2% has been approved for the treatment of atopic dermatitis in the USA since 2016 and in the EU since 2020. Efficacy and safety of the steroid-free, topical PDE-4 inhibitor have been demonstrated in two phase III studies [6]. The anti-inflammatory effects are due to the inhibition of the enzyme phosphodiesterase-4, resulting in an increase in intracellular cAMP and reduced formation of pro-inflammatory cytokines. The ointment is applied in the morning and evening. The most common adverse effects include pain at the application site. In an indirect comparison (NMA*) with pimecrolimus 1% and tacrolimus 0.03%, crisaborole 2% showed comparable efficacy in patients aged ≥2 years with mild to moderate atopic dermatitis [7]. And a recently published matching-adjusted indirect comparison (MAIC) suggests that the likelihood of achieving an improvement in ISGA score is greater with crisaborole [8].
#
is greater with crisaborole [8].
* NMA=Network meta-analysis
# ISGA=Investigator’s Static Global Assessment, (ISGA 0/1=”appearance-free or nearly appearance-free”).
Other topically applicable PDE-4 inhibitors are in clinical development, including Difamilast and Roflumilast. In a phase III study published in the Journal of the American Academy of Dermatology, Difamilast ointment 1% was shown to be superior to vehicle control in adult Japanese patients with atopic dermatitis with respect to Investigator Global Assessment (IGA) scores [9]. With regard to Roflumilast cream, the results of the Phase III studies INTEGUMENT 1 and 2 (“INterventional Trial EvaluatinG roflUMilast cream for the treatmENt of aTopic dermatitis”) are expected towards the end of 2022 [10]. According to a meta-analysis based on several clinical trials, roflumilast is an effective and safe treatment option for mild to moderate atopic dermatitis [11]. Current data suggest that roflumilast has a stronger antipruritic effect than the previously approved PDE-4 inhibitors.
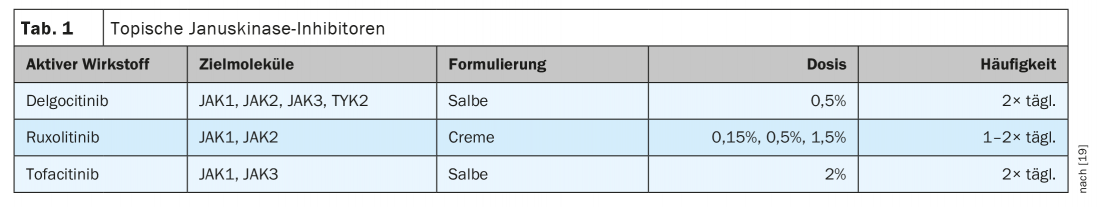
Janus kinase inhibitors: ruxolitinib, tofacitinib, and delgocitinib.
In the USA, a cream containing the active ingredient ruxolitinib has been approved for the treatment of atopic dermatitis since 2021. Two phase III studies, TRuE-AD (“Topical Ruxolitinib Evaluation in Atopic Dermatitis Study”)-1 and TRuE-AD-2, evaluated the efficacy and safety of ruxolitinib cream in patients over 12 years of age with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3-20% affected body surface area. Randomized were 631 and 618 patients, respectively [12,13]. At week 8, significantly more patients receiving ruxolitinib cream 1.5% compared with vehicle achieved IGA-TS** (53.8/51.%/51,3% and 15%/7,6%1/7%/7,6%, respectively; p<0.0001). Significant reductions in itch manifested with ruxolitinib as early as within 12 hours of initial use (p<0.05). Ruxolitinib selectively inhibits JAK1 and JAK2. Ruxolitinib 1.5% twice daily dosing has generally been well tolerated, with few clinically relevant local reactions [12,13].
** mEASI=EASI score modified (head and neck region excluded from calculation).
Delgocitinib inhibits JAK1, JAK2, JAK3, and TYK2 and has been approved in topical dosage form in Japan for the indication of atopic dermatitis since 2020 [14]. A double-blind, vehicle-controlled phase III study evaluated delgocitinib ointment 0.5% in patients with moderate to severe atopic dermatitis [15]. A total of 158 patients aged ≥16 years applied the topicals/vehicles, at an application frequency of twice daily for 4 weeks (verum arm: n=106, vehicle arm: n=52). The reduction in mEASI** was significantly greater in the drug group (-44.3% vs. 1.7%). In addition, 51.9% achieved at least a 50% improvement in mEASI (mEASI-50) with delgocitinib, compared with 11.5% in the vehicle group. Itching was also significantly reduced after just one day. In a 24-week extension phase, all patients received delgocitinib. After 24 weeks of therapy, the mEASI-50 was 95% and the mEASI-75 was 49%. Three patients developed delgocitinib-associated eczema herpeticum during the study. Lymphopenias have not been reported.
Topical application of tofacitinib ointment 2% was studied in a phase IIa study of 69 adult patients with mild to moderate atopic dermatitis [16]. Tofacitinib selectively inhibits JAK1 and JAK3. After twice-daily use for 4 weeks, there was an 81.7% improvement in EASI compared with 29.9% in the vehicle arm. In this proof-of-concept study, 12 adverse events (AEs) occurred in the tofacitinib group compared with 26 in the vehicle group. None of the AEs were severe.
Aryl hydrocarbon receptor agonists
Earlier, preparations containing tar were used for the treatment of chronic inflammatory skin diseases [17]. As is now known, the aryl hydrocarbon receptor (AHR) is a receptor for tar components. Tapinarof is an aryl hydrocarbon receptor agonist which has shown promising therapeutic potential. Tapinarof has recently been approved in the USA for the external treatment of plaque psoriasis, and this topical aryl hydrocarbon receptor agonist has already been successfully used in a proof-of-concept study in atopic dermatitis. In the double-blind, randomized phase IIb study, adolescents and adults with atopic dermatitis were treated with tapinarof cream 0.5%, 1%, or vehicle once or twice daily for a period of 12 weeks [18]. This was followed by a 4-week follow-up period. A total of 191 of 247 randomized patients completed the study. Tapinarof treatment proved superior at week 12 in terms of both IGA response and ≥75/90 improvement in EASI scores since baseline. In addition, a higher proportion of study participants in the tapinarof group reported very or moderately severe itch relief than in the vehicle group. In general, the aryl hydrocarbon receptor agonist proved to be well tolerated, and most of the adverse events were mild or moderate.
Congress: Skin Inflammation & Psoriasis International Network
Literature:
- SPIN 2022, www.spin2022.com (last accessed 07/08/2022).
- “Topical therapy of atopic dermatitis- state of art and new players,” Razvigor Darlenski, MD, SPIN, Congress of the Skin Inflammation & Psoriasis International Network, July 08, 2022.
- Li AW, Yin ES, Antaya RJ: JAMA Dermatol 2017; 153(10): 1036-1042.
- Drug Information, www.swissmedicinfo.ch, (last accessed 07/08/2022).
- Paller AS, et al: J Am Acad Dermatol 2020; 83(2): 375-381.
- Paller AS, et al: J Am Acad Dermatol 2016;75(3): 494-503.e6.
- Fahrbach K, et al:. Dermatol Ther (Heidelb) 2020; 10(4): 681-694.
- Thom H, et al: Dermatol Ther (Heidelb) 2022; 12(1): 185-194.
- Saeki H, et al: J Am Acad Dermatol. Published online October 25, 2021. doi:10.1016/j.jaad.2021.10.027
- Topical Roflumilast Cream, www.arcutis.com/pipeline/topical-roflumilast-cream, (last accessed 07/08/2022).
- Yang H, et al: JAMA Dermatol 2019; 155(5): 585-593.
- Kim BS, et al: J Allergy Clin Immunol 2020; 145(2): 572-582.
- Bissonnette R, et al: Am J Clin Dermatol 2022; 23(3): 355-364.
- Dhillon S, Delgocitinib: Drugs 2020; 80(6): 609-615.
- Nakagawa H, et al: J Am Acad Dermatol 2020; 82: 823-831.
- Bissonnette R, et al: Br J Dermatol 2016; 175(5): 902-911.
- Merk HF: Dermatologist 2019; 70(12): 942-947.
- Paller AS, et al: J Am Acad Dermatol 2021; 84(3): 632-638.
- Traidl S, Freimooser S, Werfel T: Allergol Select 2021; 5: 293-304.
DERMATOLOGIE PRAXIS 2022; 32(4): 25-26