A number of interactions occur with the St. John’s wort preparations now increasingly used to treat mild to moderate depression. However, most of them affect specific patient groups. A critical evaluation is given in the sequel.
Medicinal preparations based on St. John’s wort extracts (Hypericum perforatum) have come under criticism in recent years due to possible interactions. This has led to the fact that in the Medicines Compendium of Switzerland (online edition 2015) the following notes are given for corresponding preparations under “Interactions”: “Interaction data indicate on the one hand an induction of the cytochrome P450 system by St. John’s wort extracts (especially CYP3A4), on the other hand an induction of transport proteins (P-glycoprotein e.g. in digoxin). This may lead to a decrease in plasma concentration and to an attenuation of the therapeutic effect of a number of comedicated drugs and, especially in the case of substances with a narrow therapeutic range, to potentially serious consequences. Plasma levels and/or effects of interacting drugs (especially those with a narrow therapeutic range) should therefore be monitored closely and their dosages adjusted at the beginning and end of therapy and when the dose of St. John’s wort preparation is changed.”
Contraindications
The following groups of preparations are also listed as absolute contraindications:
- Immunosuppressants
- Anti-retroviral agents from the group of non-nucleoside reverse transcriptase inhibitors and proteinase inhibitors.
- Cytostatic drugs (imatinib, irinotecan)
- Coumarin-type anticoagulants.
The following substances/substance groups are mentioned in the relative contraindications:
- Antidepressants and other serotoninergic substances
- Digoxin
- Methadone
- Hormonal contraceptives.
Regarding hormonal contraceptives, it is stated, “Thus, several cases of intermenstrual bleeding under low-dose so-called micropills (content of ethinyl estradiol 30 μg or less) have been reported internationally. Cases of unwanted pregnancies with the use of hormonal contraceptives and concomitant use of St. John’s wort extracts have also been reported.”
Among the contraindications, “known light hypersensitivity” is also mentioned, which always gives rise to discussions in doctors’ offices and pharmacies.
Critical evaluation of the current state of knowledge is worthwhile
These many possible interactions, which have led to numerous absolute and relative contraindications, are repeatedly cited to indicate the danger or precariousness of St. John’s wort preparations.
There was also a call for all St. John’s wort preparations to be made prescription-only. The possible interactions of Hyperi-cum perforatum are in no way to be minimized here, but may be reflected upon again in a scientifically clean manner.
Close medical control
It goes without saying that patients taking immunosuppressants, anti-retrovirals, cytostatics, anticoagulants and digoxin must be under constant and close medical supervision. They also know not to take any other medications without consulting them. Furthermore, it is the duty of pharmacists to draw attention to the mentioned possible interactions when selling St. John’s wort preparations.
Herbal preparations are also drugs
When informing such patients, not only should the use of other medications be simply prohibited, but it should be pointed out that this includes herbal preparations. It is a well-known phenomenon that patients assure their doctors that they are not taking any other medications, but in the same breath point out that they are taking herbal preparations that they do not consider to be medications at all. Whether this is a herbal preparation registered as a drug or one of the increasing number of herbal supplements appearing on the market remains to be seen. A St. John’s wort preparation should not be taken by the patient groups mentioned here in their own decision.
Light dermatoses
The fact that the intake of St. John’s wort preparations can lead to light dermatoses in certain individuals seems to be one of the statements that are repeated again and again without there being any really robust scientific justification for it.
Possibly this goes back to the so-called hypericism, which is understood to be skin burns in grazing animals that had eaten a large amount of St. John’s wort. However, this was only observed in particularly susceptible animals. Scientific studies [1] were able to hold hypericin and pseudohypericin, both ingredients of St. John’s wort, responsible for these complaints. In humans, such light dermatoses have only been observed with repeated administration of hypericin-containing preparations at concentrations used in the antiviral treatment of HIV patients. At 2000 mg/d, these are far above the usual concentrations present in a “normal” St. John’s wort preparation. In the cited study by Schulz et al. the Hypericum preparation STW3 was administered to volunteers at concentrations of 612 and 900 mg. No signs of light dermatosis were observed in any of the subjects. Also, according to the ESCOP monograph “Hyperici Herba”, photosensitivity is not increased in patients taking therapeutic doses of St. John’s wort extracts [2].
Oral contraception
A possible reduction in the efficacy of oral contraceptives due to concomitant use of St. John’s wort preparations is the only interaction of Hypericum perforatum that is relevant to broad populations.
Clinical studies [3,4] have shown that appropriate preparations significantly decreased plasma levels of both hormones in women taking an oral contraceptive containing norethindrone and ethinyl estradiol. In the study by Hall et al. [3], interstitial bleeding was observed in two out of seven women. However, serum concentrations of follicle-stimulating hormone, luteinizing hormone, and progesterone were not significantly altered. The interstitial bleeding appears to be due to induction of CYP 450 isoenzyme 3A by Hypericum perforatum preparations.
In a study by Pfrunder et al. [5], no evidence was found that concomitant use of hormonal contraceptives and St. John’s wort preparations resulted in adverse ovulations.
Schulz [6] pointed out in an article that 20-60% of all women taking low-dose contraceptives containing ethinyl estradiol report intermittent bleeding.
Contraception is basically a very sensitive topic, which is sometimes also discussed very emotionally. Therefore, despite the data that clearly speak against unwanted ovulations with the associated risk of unwanted pregnancies, great caution is advised when taking oral contraceptives and St. John’s wort preparations at the same time. And therefore it is recommended to use a second contraceptive in this case.
Summary
Most interactions of Hypericum perforatum preparations involve special groups of patients who, because of the severity of their disease, are under close medical supervision.
control. Accidental ingestion is therefore very unlikely. Nevertheless, medical personnel, i.e. physicians and pharmacists, must point out the possible interactions in appropriate situations. An increase in photosensitivity by therapeutic doses of St. John’s wort extracts does not seem to exist according to current knowledge. Because of the probable increase in interstitial bleeding in the case of simultaneous use of oral contraceptives and St. John’s wort preparations, an additional contraceptive should be used here.
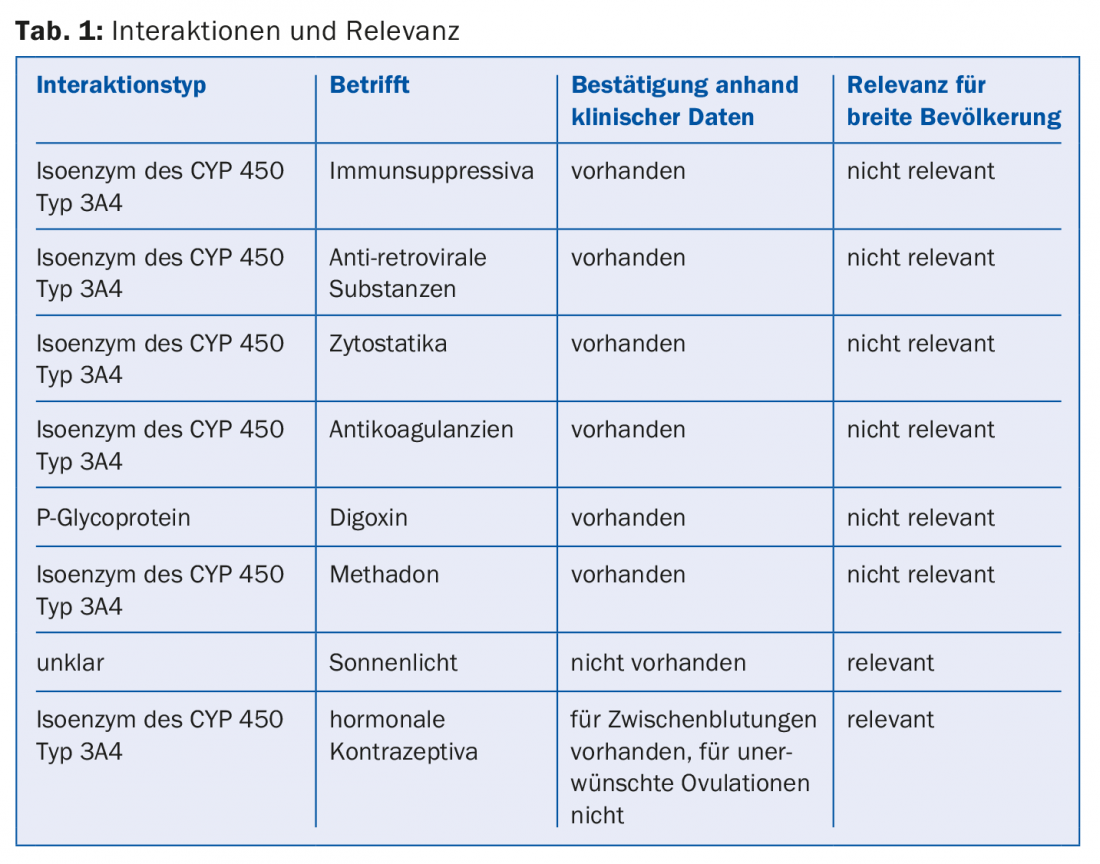
Table 1 summarizes the various interactions again.
Conclusion
Despite the interactions discussed here, Hypericum perforatum remains a safe drug for most individuals to use without hesitation, provided the precautions mentioned above are observed and appropriate education is provided by professionals.
Literature:
- Schulz, HU, et al: Investigation of the Effect on Photosensitivity following Multiple Oral Dosing of Two Different Hypericum Extracts in Healthy Men. Arznei-Forsch/Drug Res 2006; 56: 212-221.
- European Scientific Cooperative on Phytotherapy (ESCOP) Monographs, Thieme, Stuttgart 2003.
- Hall SD, et al: The interaction between St. John’s wort and an oral contraceptive. Clin Pharmacol Ther 2003; 74(6): 525-535.
- Murphy PA, et al: Interaction of St. John’s wort with oral contraceptives. Contraception 2005; 71(6): 402-408.
- Pfrunder A, et al: Interaction of St. John’s wort with low-dose oral contraceptive therapy: a randomized controlled trial. J Clinic Pharmacol 2003; 56: 683-690.
- Schulz V: No reversal of the contraceptive effect of two ovulation inhibitors by St. John’s wort extract. Journal of Phytotherapy 2004; 25(6): 301-302.
HAUSARZT PRAXIS 2015; 10(9): 2-4