Worldwide, iron deficiency is the most common deficiency disease. In Europe, the prevalence is 5-10% and is one of the most common causes of anemia, accounting for about 50%. An imbalance between demand and uptake initially leads to a storage iron deficiency; if there is an insufficient supply of the erythropoietic precursors, this leads to iron-deficient erythropoiesis, while hemoglobin is still normal. Only when the hemoglobin falls below the normal values does one speak of iron deficiency anemia.
Worldwide, iron deficiency is the most common deficiency disease. In Europe, the prevalence is 5 -10% and is one of the most common causes of anemia, accounting for about 50%. Iron deficiency is divided into three stages. An imbalance between demand and intake initially leads to a storage iron deficiency (stage I), if there is an insufficient supply of the erythropoietic precursors, this leads to iron-deficient erythropoiesis (stage II), with hemoglobin still normal. Only when the hemoglobin falls below the normal values (adult men: 13 g/dl, women: 12 g/dl) is it referred to as iron deficiency anemia (stage III). This staging is primarily focused on erythropoiesis, however, each of our cells requires iron. Erythropoiesis is preferentially supplied with iron deficiency, but this also means that iron is already lacking in other systems before anemia is present. Already in stage I and II iron deficiency related disorders can occur.
Physiology and pathophysiology of iron metabolism
The body of a healthy adult contains 3 -5 g of iron, most of which is in the form of hemoglobin iron, another portion as storage iron (adult men 500 -1000 mg, premenopausal women 200 – 400 mg) and only a very small portion in the form of plasma iron. Our body obtains the bulk of its daily iron requirements by recycling from internal iron stores, but relies on enteral absorption to avoid a negative balance. In a normal, balanced diet, the average absorption of 1-2 mg/day (about 5 -10% of the dietary iron in a normal mixed diet) compensates for physiological loss. In menstruating women, iron balance often becomes negative with daily iron loss up to 3 mg. To maintain iron homeostasis, a daily intake of 8 mg (men) and 18 mg (women of childbearing age) is required because of only partial iron absorption [1]. Most of the dietary iron is excreted in the stool.
Our food contains iron in different forms. While the iron from meat consists largely of bivalent heme iron, it is present in vegetables and cereals in trivalent form. Absorption occurs predominantly in the duodenum. Meat-derived heme iron is taken up by a receptor (heme carrier protein 1) on the surface of enterocytes. Intracellularly, iron is cleaved by a heme oxygenase and bound to mobilferrin. From here, it can be used for cellular processes or released into the blood via ferroportin 1 at the basal membrane of enterocytes. Absorption of heme iron is less susceptible to disruption and more effective than absorption of iron from vegetables and grain products. Trivalent iron must first be converted to divalent iron by DCYTB (duodenal cytochrome B). Uptake into enterocytes occurs via DMT-1 (divalent metal transporter 1), a special pH-dependent iron transporter. This process can be impeded by numerous substances (including antacids, calcium, oxalates, phosphates). From enterocytes, divalent iron also enters the bloodstream via ferroportin 1-. Before Release into the portal blood, the bivalent iron is oxidized into trivalent by hephaestin or coeruloplasmin and bound to the apo-transferrin formed in the liver (apo-transferrin+2Fe3+ -> transferrin). Under normal conditions, 16 – 45% of transferrin molecules in plasma are saturated with iron. Uptake into target cells occurs via transferrin receptors (TfR), whose number on the cell surface is regulated by the iron demand of the respective cell.
Iron storage is predominantly through ferritin, which is found in all body cells and fluids. It represents a very rapidly available reserve, and its serum concentration correlates well with available iron stores in healthy individuals. The regulation of iron absorption from food occurs via a peptide hormone formed in the liver, hepcidin. It leads to downregulation of DMT-1 and degradation of ferroportin 1. Hepcidin elevation causes decreased uptake from food as well as decreased release from enterocytes into portal blood.
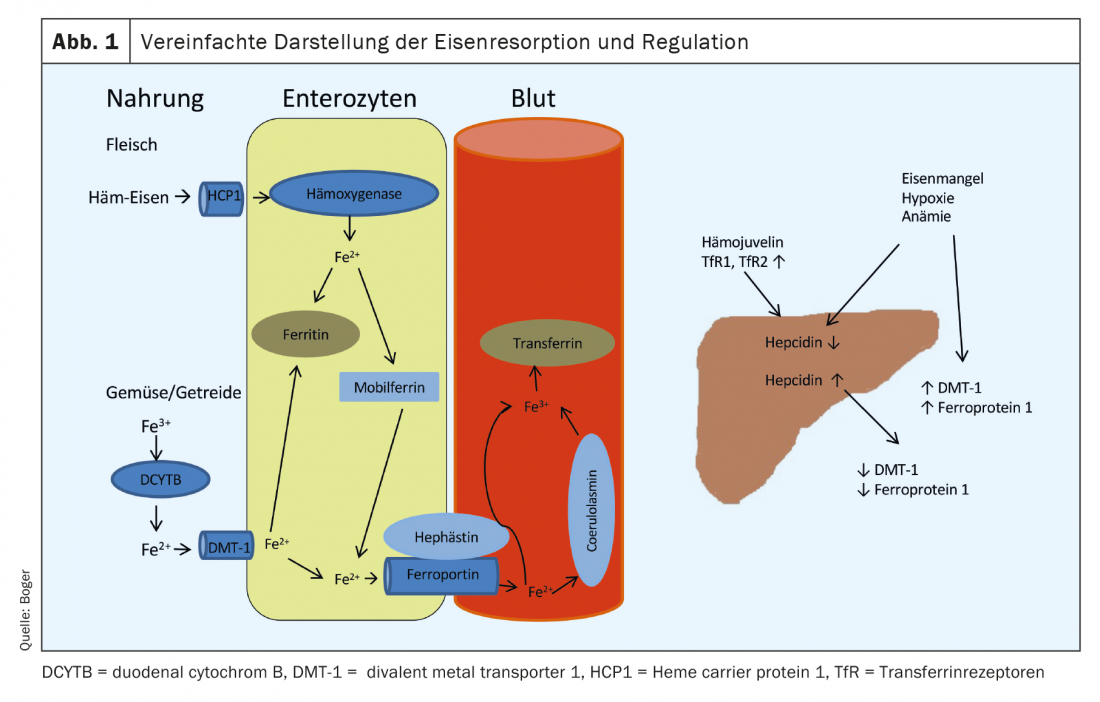
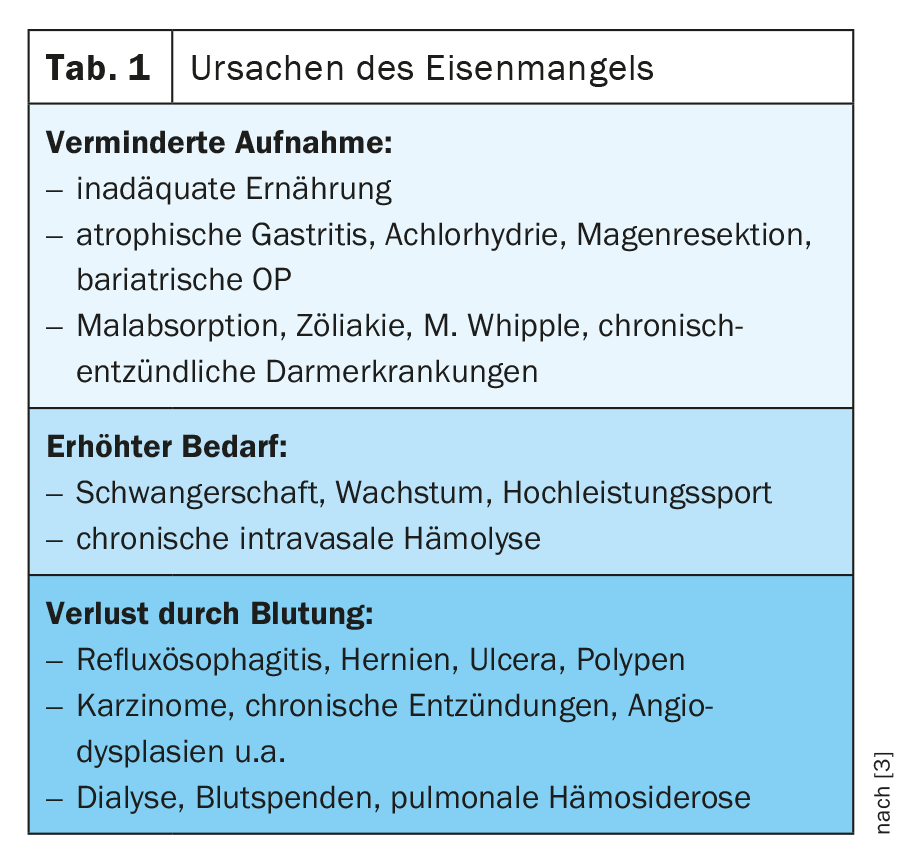
If anemia, iron deficiency, or hypoxia is present, hepcidin production in the liver is decreased and expression of DMT-1, DCYTB, hephestin, ferroportin 1, and HCP1 is increased in enterocytes [1] (Fig. 1). An overview of causes of iron deficiency is given in Table 1 .
Functional iron deficiency
For intrinsic iron utilization disorders, the term functional iron deficiency has gained acceptance in recent years. The term was first developed for renal anemia, but today the term also encompasses anemia associated with chronic diseases (tumors, infections, autoimmune diseases). The release of inflammatory cytokines (e.g. interleukin-1α, interleukin-1β, interleukin-6, tumor necrosis factor-α) leads to the induction of hepcidin, which results in reduced resorption and impaired internal transfer to the transport protein transferrin and thus explains the reduced transferrin saturation (TSAT) as an indicative diagnostic laboratory finding.
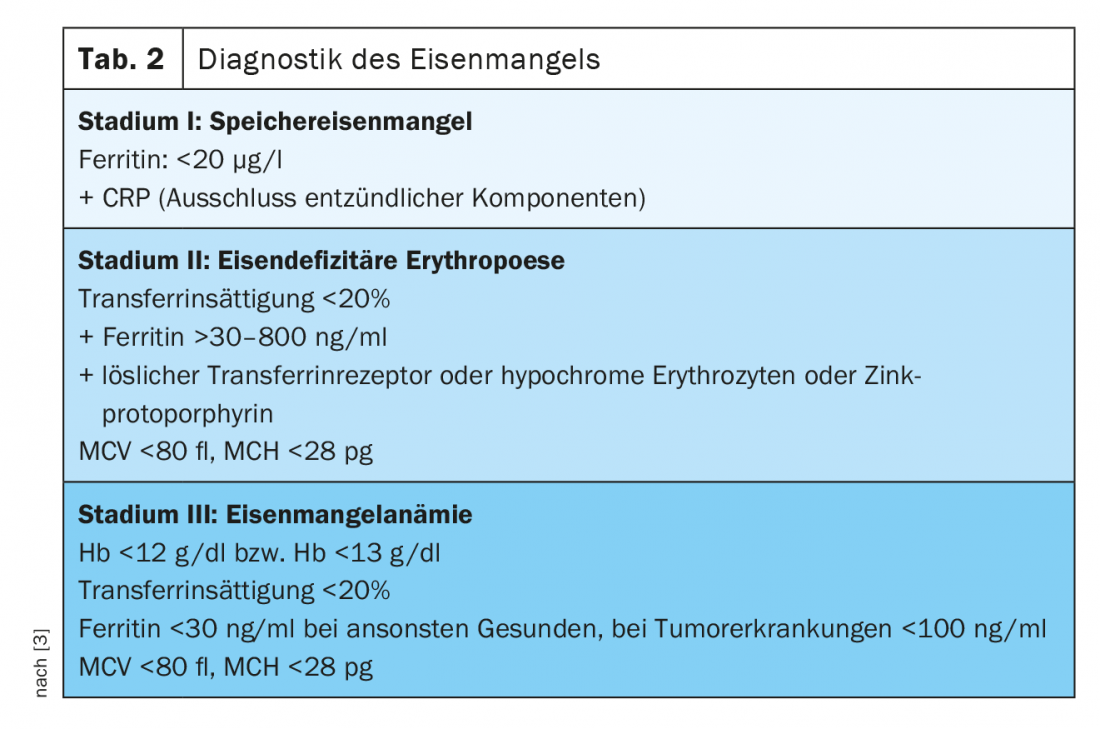
Iron deficiency diagnostics
Stage I (storage iron deficiency)
Serum ferritin correlates with the status of iron stores in healthy individuals and can diagnose iron deficiency as early as -stage I. While healthy individuals are said to be iron deficient at levels below 20 µg/l, we know that patients with solid neoplasms and proven iron deficiency may have ferritin levels of 100 – 800 µg/l, and in a quarter of patients even above 800 µg/l [2].
Although Berliner blue staining of the bone marrow smear based on the proportion of sideroblasts (erythroblasts with evidence of intracellular iron granules) provides an excellent assessment of iron content, this invasive diagnostic technique is not the method of choice for simple iron deficiency. However, if a puncture is performed in oncological patients in particular due to a different issue, this staining can be additionally performed if the cause of anemia is unclear.
Stage II (iron-deficient erythropoiesis)
Transferrin saturation (TSAT) as a measure of available functional iron is subject to circadian variation and may be decreased in inflammatory processes despite normal ferritin levels. It is a parameter of functional iron deficiency (TSAT [%]=serum iron [µg/dl]/serum transferrin [mg/dl]×70.9). Under physiological conditions, up to 45% of transferrin molecules are loaded with iron. If the percentage is below 20%, an iron deficiency situation must be assumed.
The concentration of soluble transferrin receptors (sTfR) in serum depends on the activity of erythropoiesis and iron status. If there is a pure storage iron deficiency, it is in the normal range. Serum levels increase in iron-deficient erythropoiesis. Thus, they serve very well as parameters for differentiating iron-deficient erythropoiesis and functional iron deficiency. The values of the soluble transferrin receptor and serum ferritin can be used to calculate the so-called TfR-F index, which allows a higher sensitivity and specificity with regard to a statement on iron-deficient erythropoiesis. Another parameter for the diagnosis of iron-deficient erythropoiesis is the percentage of hypochromic reticulocytes (HYPO). With adequate iron supply or stage I iron deficiency, the percentage of hypochromic erythrocytes is less than 2.5%. If this is above 10%, this is evidence of iron-deficient erythropoiesis. The determination of the hemoglobin content of reticulocytes (CHr) is also a very early parameter of iron-deficient erythropoiesis.
A parameter not yet widely used in everyday practice is zinc protoporphyrin (ZPP). If no iron is available during heme synthesis, zinc is metabolized instead and ZPP globin is formed. ZPP is formed from stage II of iron deficiency. A very inexpensive measurement by hematofluorometry, which can be used as a point-of-care diagnostic, allows diagnosis and quantification of iron-deficient erythropoiesis, although different measurement methods and lack of standardization hamper its widespread implementation [3]. Measurement of hepcidin also allows differentiation of absolute iron deficiency from functional iron deficiency in the context of chronic diseases. In the absence of standardization, this parameter is also not used in everyday clinical practice.
In stage III , with an Hb concentration of <12 g/dl in women or <13 g/dl in men, manifest anemia with hypochromic (MCH <28 pg) and microcytic erythrocytes (MCV <80 fl) occurs. It is not uncommon to find reactive thrombocytosis as an expression of the cross-reactivity of thrombopoiesis to increased erythropoietin levels (for an overview of the diagnostics of iron deficiency, see Table 2).
The specific aspects of the oncological patient
In oncological patients, absolute and functional iron deficiency is often found superimposed due to inflammation, disturbed nutrition and blood loss or shortened erythrocyte survival time (Fig. 2).The German S3 guideline Supportive Therapy in Cancer Patients recommends an appropriate diagnosis already at initial diagnosis. Other, non-tumor-specific causes of anemia must also be clarified [4]. An algorithm for anemia diagnosis is compiled in Figure 3.
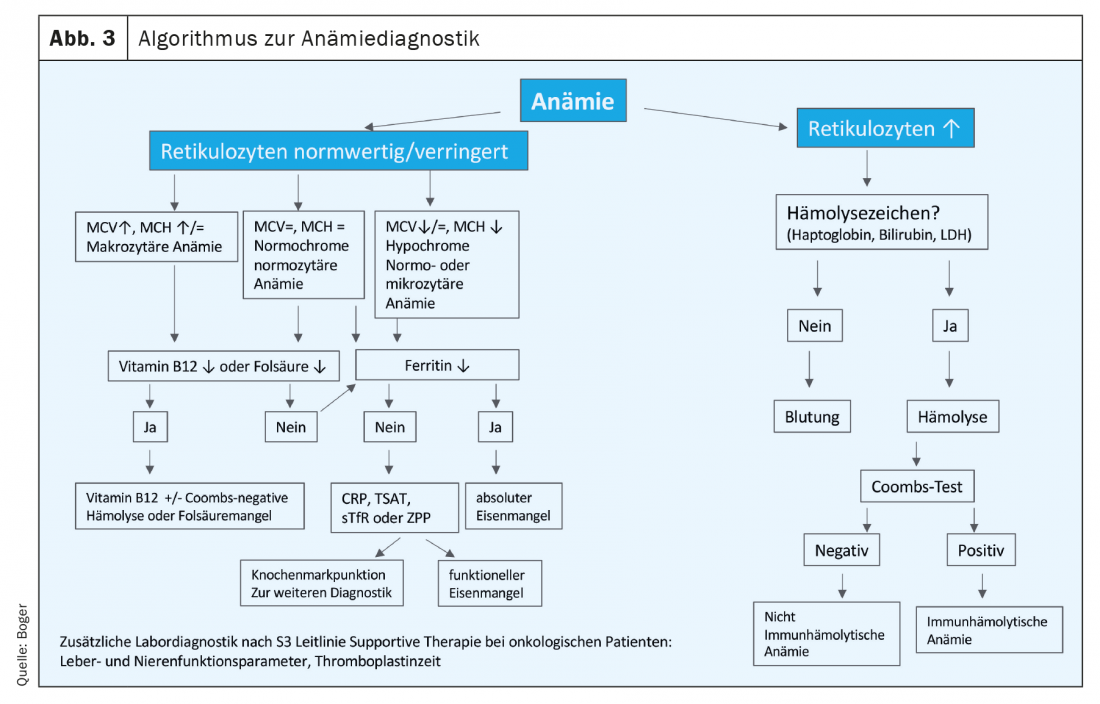
Already at diagnosis, half of all tumor patients have both anemia and iron deficiency. Under therapy, the numbers increase significantly. Not all tumors cause anemia or iron deficiency to the same extent. In Figure 4 provides an overview of the prevalence in different tumor diseases [5,6].
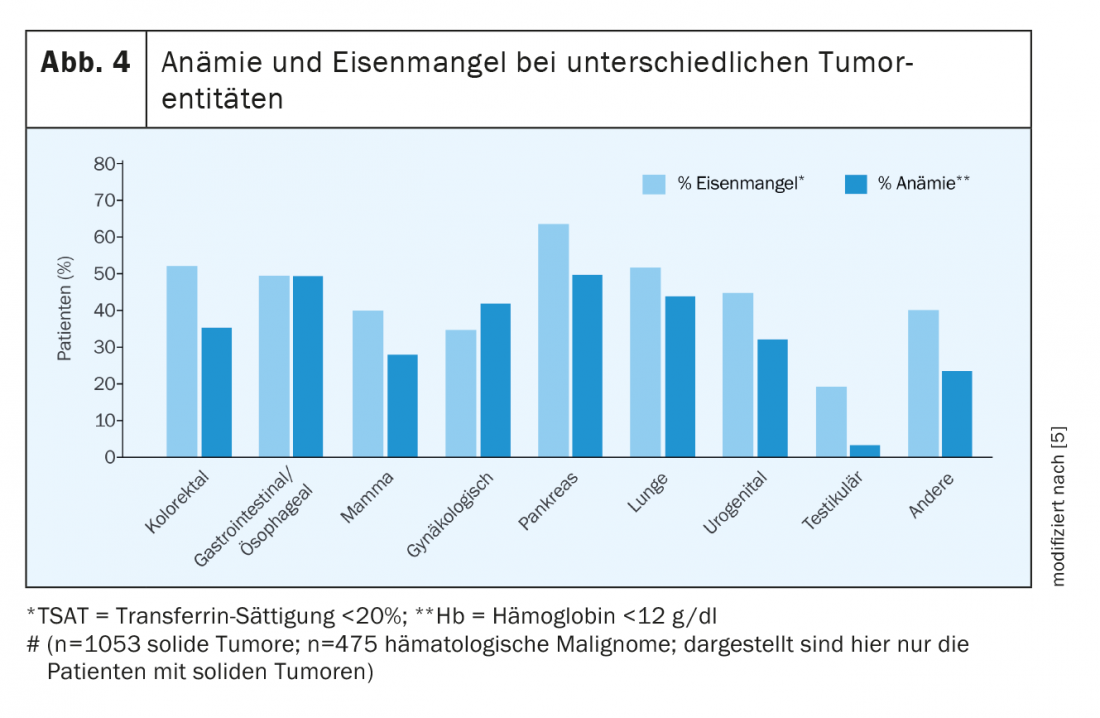
Chronic bleeding is particularly common in gastrointestinal or gynecologic tumors. Blood loss stimulates hyperregenerative erythropoiesis via reactive erythropoietin induction, resulting in increased iron requirements. If, in addition, a functional iron deficiency is present in chronic inflammation and, furthermore, erythropoiesis is inhibited by medical tumor therapy or radiation, erythropoietin is frequently elevated in normal renal function, but the increase is not sufficient to compensate for the multicausal anemia.
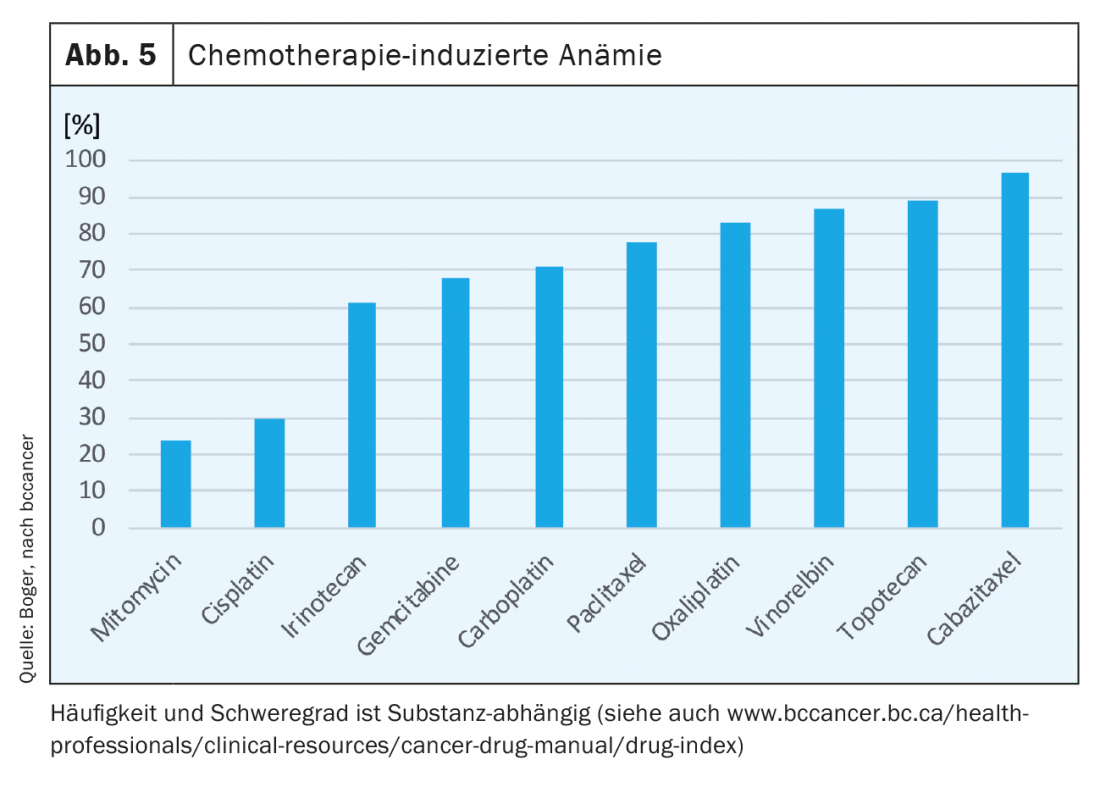
Anemia occurs in up to 75% of all oncology patients during the course of treatment. With radiotherapy alone, depending on tumor entity, in up to 50% of patients. Chemotherapeutic agents suppress erythropoiesis and, depending on the agent, cause anemia (Fig. 5) . Under combination therapies we see an additive effect and also with number of cycles there is often an increase in anemia [7].
Therapy of iron deficiency and iron-deficient anemia in tumor patients.
Iron substitution
Absolute iron deficiency is a clear indication for substitution. For the calculation of the iron requirement, the calculation according to Ganzoni has become established:
|
Total iron deficit (mg) = [Soll-Hb – Patienten Hb (g/dl)] × body weight (kg) × 2.4 + storage iron* (mg). * |
Oral substitution
For oral substitution, preparations containing both bivalent and trivalent iron are available. Dosages range up to 200 mg daily. Even in case of very good tolerance, the organism can take max. 10% of which is absorbed. Oncological patients often have a very high demand and usually a functional iron deficiency, which is why oral substitution causes unnecessary side effects (gastrointestinal tolerance) when absorption is impaired , without efficiently replenishing iron stores. Before deciding on oral substitution therapy, the patient’s inflammation status and other possible contraindications to oral substitution (e.g., gastric resection, malabsorption disorders, chronic constipation) must be determined. Constipation, impaired adherence to therapy).
Frequently used substances in oral supplementation are ferrous fumarate, ferrous gluconate and ferrous sulfate. Recently, alternative substances with improved absorption potential and more favorable tolerability profile (but less favorable cost profile) have appeared on the market: ferric maltol polysaccharide complexes and liposomal ferric formulations [8].
As early as 14 days after initiation of substitution, there should be a hemoglobin increase of ≥1 g/dL. If this is not the case, a switch to an intravenous preparation should be made [9]. After normalization of hemoglobin levels, oral supplementation should be continued for at least three months to replenish iron stores.
Intravenous substitution
Intravenous substitution should be preferred in tumor patients with proven iron utilization disorders and iron-deficient erythropoiesis or iron deficiency anemia. Especially in the case of concomitant therapy with erythropoiesis-stimulating substances, the preferential use of intravenous formulations for efficient supplementation is warranted [3].
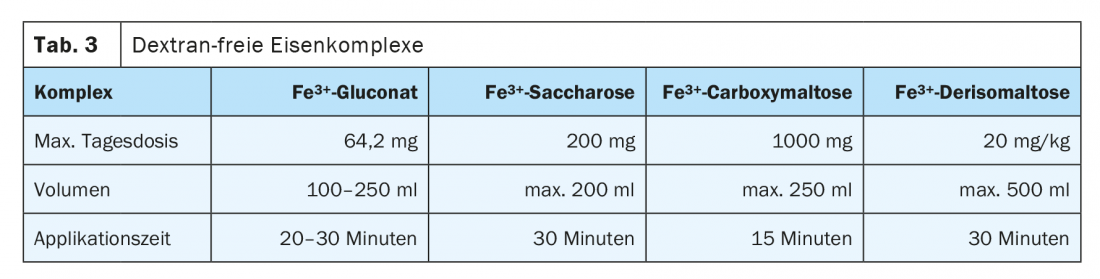
There is a risk of anaphylactic reaction with all parenteral iron preparations because of the Fe-binding partners, but this is very low with modern substances. As early as 2013, the European Medicines Agency (EMA) determined in a risk assessment procedure that the benefits outweighed their potential risks. The risk is higher with preparations containing dextran, so dextran-free preparations are to be preferred. A significantly more favorable side effect profile exists for ferric gluconate, ferric hydroxide sucrose, and ferric carboxymaltose. These are colloidally dissolved nanoparticles. These are taken up and degraded in the liver and spleen by the reticuloendothelial system. The released iron is stored or distributed in the organism by transferrin. Intravenous administration should preferably be given as a short infusion. Too rapid application may exceed the transferrin binding capacity and cause flushing symptoms. The application volume, the amount of iron bound in complex per infusion and single vs. multiple dosing differ significantly and determine therapy frequency and control intervals. Table 3 lists several dextran-free preparations that can be administered intravenously.
Transfusion of red cell concentrates
The indication for transfusion is based on clinical condition, patient risk factors, severity of anemia symptoms, Hb level (or hematocrit), acute nature of blood loss, and compensation options [4]. In patients with acute blood loss, solid tumor, or hemoblastosis, transfusion indication must be evaluated starting with a hemoglobin value ≤8 g/dL. Patients with chronic anemia sometimes have Hb values between 6 – 8 g/dl without any symptoms and thus no compelling transfusion indication. In patients with concomitant cardiac or pulmonary disease, hemoglobin should be stabilized at 10 g/dL.
Therapy with erythropoiesis-stimulating agents
In chemotherapy-induced anemia, erythropoiesis-stimulating agents (ESAs) are approved for symptomatic anemias with an Hb value -≤10 g/dL [4]. The combination of ESA and intravenous iron supplementation during chemotherapy can significantly reduce the number of blood transfusions [10]. Timing of iron supplementation should precede ESA therapy. However, careful consideration of the risks with respect to thrombophilic risk profile and individual benefit of ESA therapy for the patient must be made [11].
Other therapeutic approaches for functional iron deficiency in tumor patients, such as androgen therapy or the use of hepcidin and bone morphogenetic protein (BMP) antagonists, are currently the subject of clinical research [12].
Summary
Iron deficiency and anemia are difficult to consider separately in oncology patients. Both anemia and iron deficiency usually have multiple underlying causes. It is therefore always necessary to exclude or treat other, non-tumor-specific causes. Most oncology patients are at risk for malnutrition, and qualified nutritional status assessment and regular nutritional counseling should be provided early during oncology treatment. However, iron deficiency occurs in most patients despite optimized oral intake. In the case of iron deficiency caused by chronic bleeding and chronic inflammation, the increased needs of the organism cannot and must not be met by oral substitution.
Chemotherapy suppresses erythropoiesis and endogenous erythropoietin release does not meet the stimulation requirement at permanent erythropoietic negative balance. Transfusion therapy should be used only when necessary for immediate anemia correction (bleeding, cardiocirculatory risk) because of toxic effects and negative prognostic potential. Intravenous iron supplementation is the preferred anemia-preventing or correcting therapy.
Take-Home Messages
- When malignant disease is first diagnosed, substrate analysis of erythropoiesis and anemia diagnostics should be performed.
- Hb, iron status (TSAT, serum ferritin), and CRP should be determined at baseline and before each chemotherapy cycle.
- Functional iron deficiency in cancer patients is characterized by impaired utilization of intrinsic iron stores.
- Oral iron should be considered only in patients with absolute iron deficiency (ferritin <30 ng/ml) and non-inflammatory condition (CRP <5 mg/l).
- Intravenous iron supplementation is the preferred iron therapy in the oncology patient.
Literature:
- Finberg KE: Regulation of systemic iron homeostasis. Current opinion in hematology 2013; 20(3): 208-214.
- Ludwig HE, et al: Iron metabolism and iron supplementation in cancer patients. Wien Klin Wochenschr 2015; 127(23-24): 907-919.
- Hastka J, et al: Iron deficiency and iron deficiency anemia. Onkopedia Lead Lines 2020; www.onkopedia.com/de/onkopedia/guidelines/eisenmangel-und-eisenmangelanaemie/@@guideline/html/index.html, accessed 06/29/2020.
- Jordan K, et al: Guideline program oncology (German Cancer Society, German Cancer Aid, AWMF): supportive therapy in oncological patients – long version 1.3.2020; www.leitlinienprogrammonkologie.de/leitlinien/supportive-therapie, accessed 29.06.2020.
- Ludwig H, et al: Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 2013; 24(7): 1886-1892.
- Ludwig H, et al: The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004; 40(15): 2293-2306.
- Xu H, et al: Incidence of anemia in patients diagnosed with solid tumors receiving chemotherapy, 2010-2013. Clin Epidemiol 2016; 8: 61-71.
- Farrag K, et al: New options for oral iron substitution. Drug Therapy 2019; 37(4): 105-112.
- Okam MM, et al: Iron Supplementation, Response in Iron-Deficiency Anemia: Analysis of Five Trials. The American journal of medicine 2017; 130(8): 991 e991-991 e998.
- Bastit L, et al: Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2008; 26(10): 1611-1618.
- Aapro M, et al: Management of anemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 2018; 29(Suppl 4): iv96-iv110.
- Gilreath JA, Rodgers GM: How I treat Cancer Anemia. Blood 2020; doi: 10.1182/blood.2019004017.
HAUSARZT PRAXIS 2020; 15(10): 6-11