Possible treatments for soft tissue sarcomas include surgery, radiation therapy, and chemotherapy. Recently, attempts have been made to enhance the effects by exposing the cancer cells to heat – with success.
In adults, soft tissue sarcomas are rare compared to other cancers (overall, they account for less than 1% of all malignant tumors). The therapy is planned individually and depends, among other things, on the localization, size of the sarcoma and metastases. Localized forms usually undergo surgery combined with pre- or postoperative radiation, and perioperative chemotherapy is also recommended as an option for high-risk patients.
“Crucible” for cancer cells
In preclinical, as well as early randomized clinical trials, synergistic effects were found when radio- and chemotherapy were combined with regional heat exposure of 40 to 43°C. The heat not only kills the cancer cells by direct thermal toxicity, but also increases the drug effect resp. sensitization of the tissue to chemo or radiation and ultimately induces an (antitumor) immune response via stress proteins and various other warning signal mechanisms [1].
A group of researchers from Munich demonstrated the feasibility and effectiveness of the procedure in high-risk sarcomas as early as 1990 [2]. Many more years went by. The first author at the time and one of the most active scientists in this field, Prof. Dr. med. Rolf D. Issels from the University Hospital in Munich, stayed on the subject. In collaboration with university centers from Norway, Austria and the USA as well as six German hospitals under recent Munich leadership, the results of a randomized phase III trial comparing regional hyperthermia in combination with neoadjuvant chemotherapy with chemotherapy alone were presented for the first time in 2010 [3].
The BSD-2000 hyperthermia system was used for this purpose. In a ring-shaped reclining device, the thermal radiofrequency energy is selectively concentrated on the target treatment region deep inside the limbs, pelvis, abdomen or thorax. The so-called phased array configuration concentrates the radiation energy by arranging individual radiators. Strong directivity is achieved with phased array antennas. At the target location, the temperature increases (water-containing tissue is heated by the coupling of electromagnetic waves), while the system suppresses the radiation elsewhere. The whole thing is monitored and controlled by computer and via sensors or “thermostat”. Target temperature in the study was 42°C (i.e., very high fever) over one hour on days 1 and 4 of each chemotherapy cycle.
Of the 341 randomized adults with localized high-risk soft tissue sarcomas, almost all underwent (re)resection and just over two-thirds per group underwent radiation. On average, patients received radiation doses of 53.2 vs. 52.7 Gy. Neoadjuvant therapy consisted of the two most active chemotherapeutic agents from this field, doxorubicin and ifosfamide. The latter is most effective at temperatures of 40.5 to 43°C. In addition, etoposide was given, which, according to the authors, could be omitted in the future because it has only a low activity in soft tissue sarcomas. The same agents were used for postinduction therapy (again with or without hyperthermia) after resection and/or radiation, which approximately half of the patients in each group underwent completely (more in the hyperthermia group).
Long-term efficacy confirmed
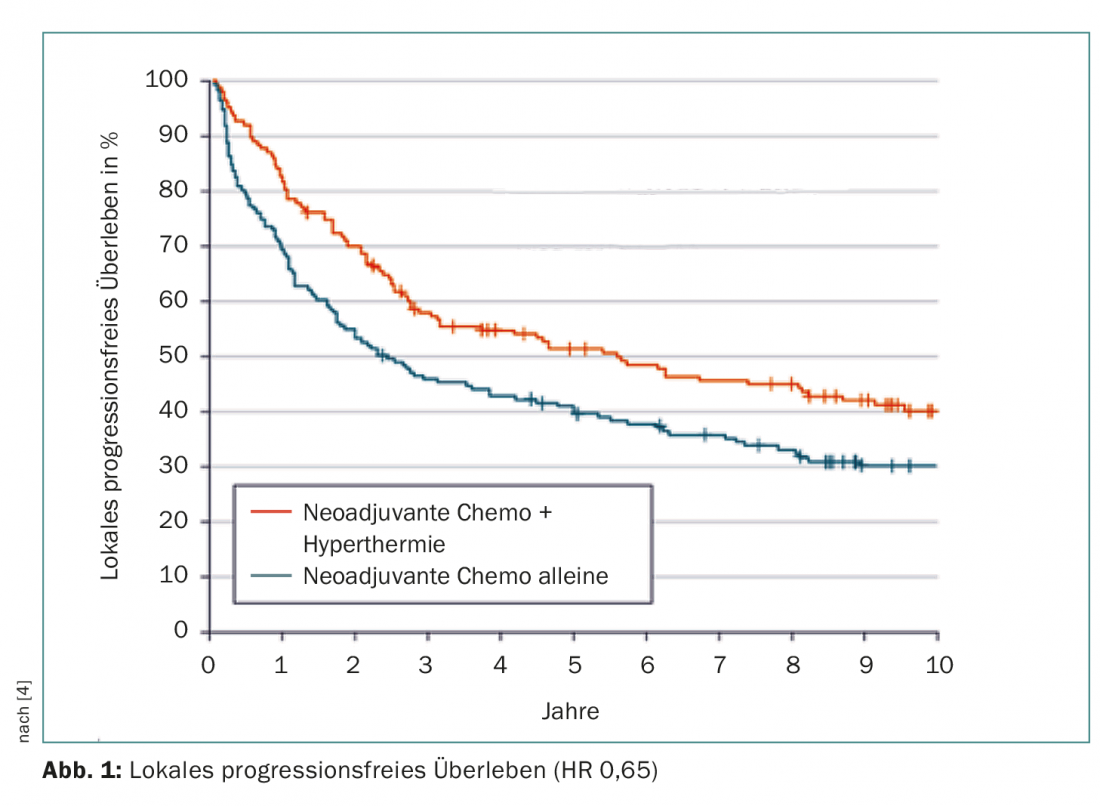
In December 2014, they completed the data collection. At this point, after a median of more than eleven years of follow-up, the addition of hyperthermia had reduced the likelihood of local progression or death by 35% (HR 0.65; 95% CI 0.49-0.86; p=0.002). This was the primary endpoint. The curves diverged rapidly from the start of the study, and early progression was effectively prevented (Fig. 1). Mortality risk was similarly improved with a significant 27% reduction in the study group (analyzing only disease- or treatment-associated cases) – namely from a good six to about 15 years. After five years, 62.7% vs. 51.3% and after ten years, 52.6% vs. 42.7% of those treated were alive. Nine patients needed to be treated with hyperthermia to save one from death in five years (Number Needed to Treat).
The long-term results [4] confirm the initial evaluation, which took place in 2010. At that time, the primary endpoint had shown a risk reduction of over 40% and a survival benefit in those who completed induction chemotherapy with hyperthermia. 28.8% versus 12.7% responded to each treatment. The review process was most effective for retroperitoneal and abdominal (non-extremity) sarcomas.
Safety acceptable
Safety-wise, the results of the combined treatment strategy were mixed. The authors spoke of “moderate toxicity,” and the treatment could generally be administered safely. The comparative therapy already represents a burden for the patients, which is only increased to a limited extent by hyperthermia. If one treatment-associated death occurred with chemotherapy alone, there were two with concurrent hyperthermia. Severe leukopenias were significantly more frequent; thrombocytopenias were also found in 17% vs. 13.8% of cases (possibly due to thermal co-treatment of the bone marrow, especially in large abdominal or pelvic tumors). Patients may have been more susceptible to such effects of chemotherapy because of the heat. Various specific side effects such as pain and varying degrees of skin burns were also seen. The pressure from the silicone/water “pillows” surrounding the patient in the applicator (designed to target the waves to the area of coverage) was sometimes found to be uncomfortable and may have promoted vomitus and local neurotoxicity in some cases, especially in patients after surgery and radiotherapy.
It remains questionable whether the risk-benefit balance of the therapeutic approach would thus also be positive in patients with lower risk. Currently, hyperthermia is also being tested in a large phase III trial in resected pancreatic cancer.
Literature:
- Issels RD: Hyperthermia adds to chemotherapy. Eur J Cancer 2008 Nov; 44(17): 2546-2554.
- Issels RD, et al: Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: a phase II study. J Clin Oncol 1990; 8(11): 1818-1829.
- Issels RD, et al: Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 2010 Jun; 11(6): 561-570.
- Issels RD, et al: Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma. The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol 2018. DOI:10.1001/jamaoncol.2017.4996 [Epub ahead of Print].
InFo ONCOLOGY & HEMATOLOGY 2018; 6(3): 5-6.