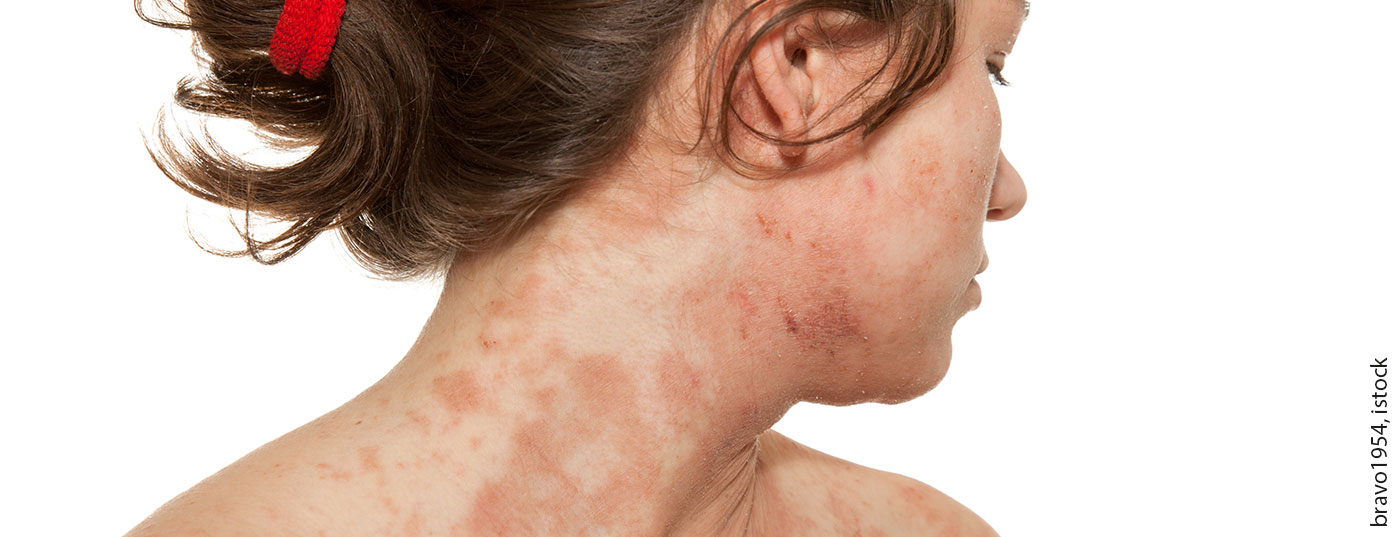
Janus kinase (JAK) inhibitors are a promising new treatment option for adult patients with atopic eczema, given their rapid relief of pruritus and inflammatory lesions. In addition to baricitinib, which has now been approved, several other JAK inhibitors are currently in advanced stages of clinical testing.
JAK inhibitors exert their symptom-relieving effect by blocking Janus kinases in their activity. “It is a small tyrosine kinase family consisting of JAK1, JAK2, JAK3, and TYK2,” explained Prof. Kamran Ghoreschi, MD, Clinic for Dermatology, Venereology, and Allergology, Charité- Universitätsmedizin Berlin, at this year’s virtual DDG meeting [1]. Janus kinases (JAKs) are enzymes that transduce intracellular signals from cell surface receptors for a number of cytokines and growth factors involved in hematopoiesis, inflammation, and immune defense [2].
Simultaneous blockade of multiple cytokine signals
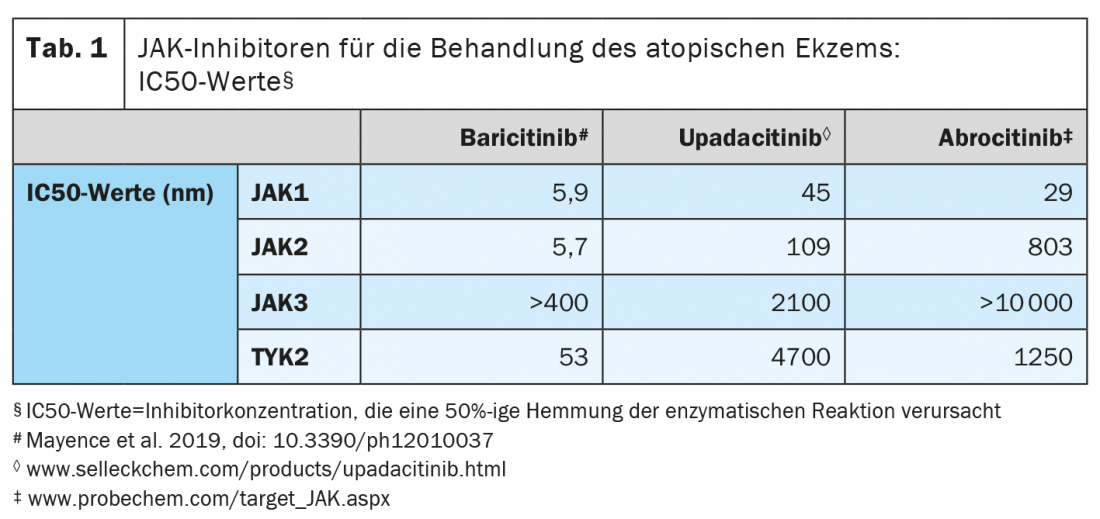
The advantage of JAK inhibitors is that inhibition of one or more Janus kinases can block numerous cytokines. Key cytokines in atopic dermatitis include IL4 and IL13, as well as IL22, IL31, IL33, and IL-TSLP and IFN-γ, explains Prof. Ghoreschi. Within the intracellular signaling pathway, JAKs phosphorylate and activate signal transducers and activators of transcription (STATs), which in turn activate gene expression within the cell. Baricitinib modulates these signaling pathways by partially inhibiting the enzymatic activity of JAK1 and JAK2, thereby reducing phosphorylation and activation of STATs. In isolated enzyme assays, baricitinib inhibited JAK1, JAK2, tyrosine kinase 2 (TYK2) and JAK3 activity with IC50 values of 5.9; 5.7; 53 and >400 nm, respectively (Table 1) . Baricitinib (Olumiant®) [3]* was the first JAK inhibitor to be approved for the treatment of adult patients with moderate to severe atopic dermatitis. Upadacitinib and abrocitinib, two additional oral JAK inhibitors, are currently being evaluated in phase III clinical trials [4].
* Swissmedic approval in February 2021
Baricitinib showed rapid and significant symptom reduction
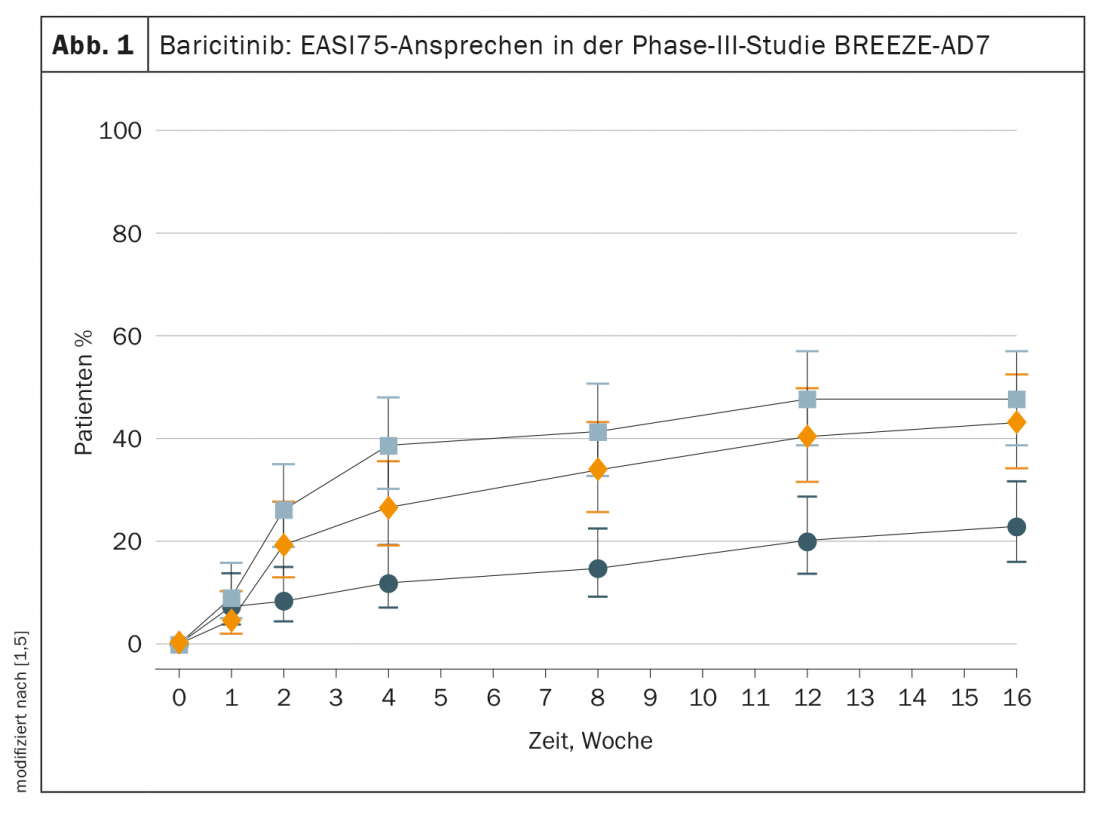
In the phase III study (n=329) of the efficacy and tolerability of baricitinib in combination with topical corticosteroids (TCS) of mild or moderate class, a significantly higher proportion of those treated with baricitinib 4 mg and TCS treated patients experienced a reduction in itch by ≥4 points on the pruritus NRS, corresponding to clinically relevant relief (p<0,001) [5]. At week 16, 44% of study participants receiving the standard dose of baricitinib and TCS achieved clinically relevant relief of pruritus, which was more than twice the proportion receiving placebo and TCS (p<0.001) [5]. EASI75 response rates over the course of the study (baseline to week 16) are shown in Figure 1.
Upadacitinib shone in Phase IIb study
The JAK-1 inhibitor upadacitinib is also currently in phase III clinical trials in adolescents and adults [6]. In the Phase IIb study (n=167), the drug demonstrated significant superiority over placebo. While 9.8% of study participants achieved an EASI75 at 16 weeks in the placebo group, 69% did so with upadacitinib 30 mg (1×/d) and 52.4% did so with upadacitinib 15 mg (1×/d). Significant improvement was also achieved in terms of itch reduction, by 48% in the lower dose (15 mg) of upadacitinib and by 68.9% in the higher dose (30 mg) compared with 9.7% in the placebo group [7]. Similar to baricitinib, the safety profile of upadacitinib has been relatively favorable to date, with comparable side effects. No increased thromboembolic events have also been reported to date [8].
Abrocitinib: Phase III data already available
Data from the phase III trial of abrocitinib, another selective JAK1 inhibitor, showed very promising results in adults and adolescents 12 years and older [6,9]. Patients received abrocitinib 100 mg, 200 mg or placebo once daily for 12 weeks. The primary endpoint, a 75% improvement in EASI, was met by 40% of study participants in the 100 mg group and 63% of those in the 200 mg group versus 12% of patients in the placebo group. In this study, there was also a rapid and significant improvement in itching. After 12 weeks, 38% of patients in the 100 mg and 57% of patients in the 200 mg group showed an improvement of at least 4 points on the itch scale compared with 15% of patients in the placebo group. The spectrum of side effects is broadly similar to that of other JAK inhibitors (susceptibility to infection, nausea, recurrent herpes (simplex) infections, and gastrointestinal symptoms) [10].
Congress: DDG Conference 2021
Literature:
- Ghoreschi K: Conventionals and innovations. S09: Track inflammation: Atopic dermatitis and psoriasis as systemic diseases. DDG Meeting 2021, 17.04.2021.
- EMA: Olumiant, Product Information, www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_de.pdf
- (last call 04/21/2021)
- Professional information Olumiant®, www.swissmedicinfo.ch, (last accessed 08.04.2021)
- Nezamololama N, et al: Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: a review of abrocitinib, baricitinib, and upadacitinib. Drugs Context. 2020; 9:2 020-8-5. doi:10.7573/dic.2020-8-5.
- Reich K, et al: Efficacy and Safety of Baricitinib Combined With Topical Corticosteroids for Treatment of Moderate to Severe Atopic Dermatitis. A Randomized Clinical Trial. JAMA Dermatol 2020; 156(12): 1333-1343.
- Quint T, Bangert C: Biologics therapy of atopic dermatitis. close up 2021; 20: 37-44.
- Guttman-Yassky E, et al: Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol 2020; 145(3): 877-884.
- Fleischmann R, et al: Upadacitinib versus placebo or ada-limumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019; 71(11): 1788-1800.
- Silverberg JI, et al: Efficacy and Safety of Abrocitinib in Patients With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020; 156(8): 863-873. doi:10.1001/jamadermatol.2020.1406.
- Simpson EL, et al: Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020; 396(10246): 255-266.
DERMATOLOGIE PRAXIS 2021; 31(3): 15-16 (published 6/1/21, ahead of print).