Although testicular cancer accounts for only 1% of oncologic disease in men, it is the most common cancer in boys. PD Dr. med. Richard Cathomas, Deputy Chief Physician at the Cantonal Hospital Graubünden, gave interesting insights into diagnostics and therapy of early stages of the disease at the Swiss Oncology and Hematology Congress SOHC.
In the western world, 3-10 out of 100,000 men are diagnosed with testicular cancer every year; in Switzerland, this still affects around 400 – mostly young – patients. While non-seminomatous tumors are also detected more frequently in those under 30 years of age, the proportion of seminomas increases in those 30-40 years of age. Overall, 95% of cases are germ cell tumors with 70% detected at stage I. The most common diagnosis is “stage I seminoma,” which occurs in about half of those affected. But what does that mean in concrete terms?
Staging and histology
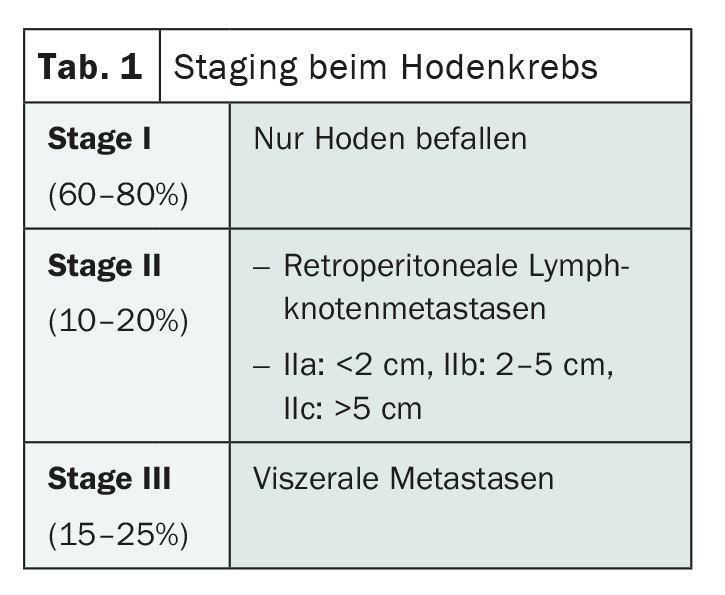
Germ cell tumors of the testis are broadly divided histologically into the two categories of seminomas and non-seminomas, with a slight preponderance of seminomas. Dr. Cathomas emphasizes that any tumor that has non-seminomatous components must be considered a non-seminoma and treated accordingly. In both groups, staging is based on the same criteria. Whereas in stage I the tumor is limited to the testes, in stage II retroperitoneal lymph nodes are affected and in stage III visceral organs are also involved, most notably the lungs (Tab.1). For a more precise subdivision and better prognosis assessment, there is a further classification for stages II and III based on the so-called IGCCCG (International Germ Cell Cancer Collaborative Group) risk groups.
Diagnostics in the confusion of guidelines
From ESMO Consensus to NCCN and EAU Guidelines, there are numerous recommendations for optimal management of testicular cancer [1–3]. At the SOHC, Dr. Cathomas presented a middle way, which – quite Swiss – represents a compromise of the different guidelines and is usually followed in this country.
In addition to anamnesis and clinical examination, the diagnostic procedure includes bilateral testicular ultrasound, determination of the tumor markers HCG, AFP and LDH, and CT of the thorax/abdomen/pelvis (overview 1) . The expert explicitly advises against performing a PET-CT. Biopsy of the unaffected side should be performed in those under 40 years of age with small testes, microlithiasis, or maldescensus to exclude simultaneous involvement of the second testis. Ideally, the histological work-up after orchiectomy takes place at a specialized center.

Postoperatively, tumor markers should be monitored until they normalize, reach a nadir, or, less favorably, rise. It is also recommended to repeat the CT after six to eight weeks in case of unclear findings before deciding on the further therapeutic approach, even if the tumor markers are in the normal range. In cases of metastatic disease and intermediate or even poor prognosis, Dr. Cathomas additionally recommends the use of a cranial MRI.
According to the expert, a chest X-ray is sufficient for radiological follow-up, although one would need to be performed only for non-seminomas. He also said that abdominal MRI is superior to CT.
One option that no patient should be deprived of is sperm cryopreservation. This possibility should be discussed at the latest before the start of radio- or chemotherapy and should be recommended to all those who wish to have a child.
Tumor markers at a glance
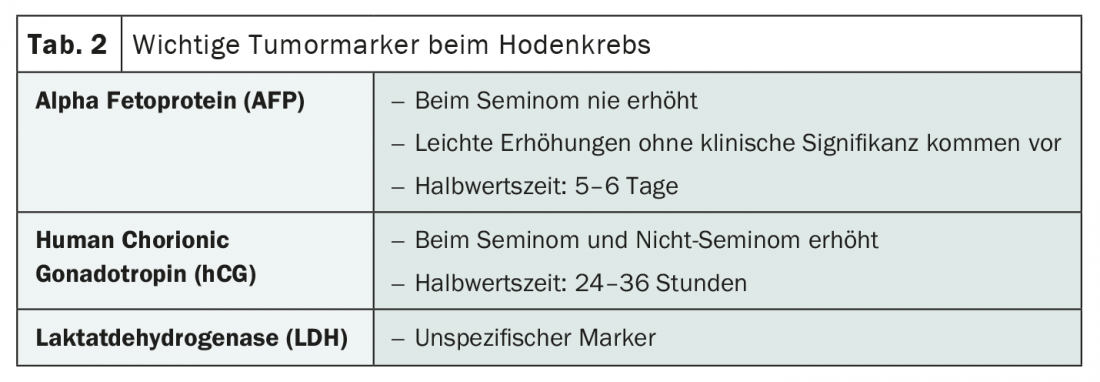
The three tumor markers alpha fetoprotein (AFP), human chorionic gonadotropin (hCG) and lactate dehydrogenase (LDH ) have long played an important role in the diagnosis and monitoring of testicular cancer (Tab. 2). Their interpretation is often sufficient to distinguish between seminomatous and non-seminomatous tumors, for example. Thus, AFP is never measurable in seminomas, whereas hCG can increase in seminomas as well as in non-seminomas. Because a slight elevation also occurs without clinical significance, caution should be exercised when interpreting AFP levels. The more nonspecific LDH must also be interpreted cautiously, but it should still be measured before starting therapy and at follow-up, according to Dr. Cathomas. Knowledge of the half-life can simplify marker design. While it is five to six days for AFP, it is 24 to 36 hours for hCG. Decisive for the risk classification according to IGCCCG are the values of the markers after orchiectomy.
Therapy of a prognostically favorable disease: focus on relapse rate
If the diagnosis confirms the most common cancer diagnosis of the testis, first-stage seminoma, the prognosis is favorable. Prerequisites for this are no metastases in staging, postoperative normalization of tumor markers, especially hCG, no AFP elevation, and no non-seminomatous tumor components. Regardless of the therapy chosen, the disease-specific 10-year survival in this case is over 99% with recurrence rates of up to 30%, with tumor size and invasion of the rete testis being unfavorable prognostic factors. However, these are not considered in the choice of treatment. Adjuvant therapy with a single dose of carboplatin AUC 7 or retroperitoneal radiotherapy can reduce the relapse rate to approximately 5%. Active surveillance after orchiectomy remains the standard of care for all patients despite this risk reduction, according to Dr. Cathomas. However, he emphasizes that adjuvant chemotherapy may be useful, especially when the tumor size exceeds 4 cm, whereas he strongly advises against the use of adjuvant radiotherapy.
If the newly diagnosed testicular tumor is a non-seminoma without metastases and with postoperative normal AFP and hCG values, the situation is somewhat different. Although the prognosis in this case is also similarly favorable as in seminoma, a subgroup exists with relapse rates of about 50%. These are tumors that show lymphovascular invasion and are referred to as high risk tumorsbecause of this characteristic. One cycle of BEP (bleomycin, etoposide, cisplatin) is recommended adjuvantly for these high-risk non-seminomatous germ cell tumors to reduce the relapse rate. This can reduce the risk of recurrence to less than 4%, according to Dr. Cathomas. However, active surveillance is sufficient for all other stage I non-seminomas, he said. Clear guidelines exist for both seminomatous and non-seminomatous germ cell tumors, which provide for regular monitoring of tumor markers and diagnostic imaging using abdominal CT or MRI, as well as chest X-rays in the case of non-seminomas, especially during the first five years.
Source: Swiss Oncology & Hematology Congress 18-21.11.2020, On Demand Session “Testis cancer: diagnostic tools and management stage I”, PD Dr. med. Richard Cathomas, Deputy Chief Physician for Oncology and Hematology at the Cantonal Hospital Graubünden.
Literature:
- Gilligan T, et al: Testicular Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019; 17(12): 1529-1554.
- Laguna M, et al: Testicular Cancer 2020. https://uroweb.org/guideline/testicular-cancer/ (last accessed 11/18/2020).
- Honecker F, et al: ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018; 29(8): 1658-1686.
InFo ONCOLOGY & HEMATOLOGY 2020; 8(6): 38-39 (published 9/12/20, ahead of print).