There is a need for improved public information and education about this disease. In industrialized countries, the rate of undiagnosed cases is estimated to be around 50%. Regarding therapy, a good cooperation between the treating ophthalmologist and the general practitioner is important.
Glaucoma is a chronic eye disease that has been known for a long time and can potentially lead to blindness. Interesting and not known to all is the historical background of the nomenclature: already the ancient Greeks noticed that certain blinded patients showed a striking color of their pupil reflex. The translation of the Greek word “Glaukos” is “gray-bluish-greenish”. The exact anatomy of the eye and the pathophysiology were not yet known. In the 8th century AD, the term “cataract” was coined for lens opacities, but still the exact mechanism of blindness was unclear. It was not until the invention of the ophthalmoscope and its ability to assess the fundus and optic nerve that the entities of cataract and glaucoma could be separated.
The term “glaucoma” used in the German language, on the other hand, was not coined until the 18th century. It was thought at the time that the “juices” in the eye could change color and would turn a greenish color in glaucoma. However, the exact origin of the term is not known. The disease can manifest at any age, but its incidence increases significantly with age. It is assumed that in Switzerland 2.5% of all persons over 40 years of age suffer from glaucoma. It is remarkable that more than half of the affected patients are unaware of their disease [1].
In 2004, glaucoma was the most common cause of irreversible blindness [2] and cataract was the most common cause of reversible blindness. Senile macular degeneration also leads to irreversible blindness. In recent years, the number of patients going blind from macular degeneration has increased dramatically due to increasing life expectancy and has taken the leading position of causes of blindness in certain countries.
Pathophysiology
In simple terms, the eyeball is a spherical structure surrounded by a solid shell. Accordingly, there is a variable internal pressure for each eye, which is important for homeostasis. This internal pressure is controlled by the ratio of aqueous humor production and aqueous humor outflow. In glaucoma, there is a mismatch of these two components, and very often the outflow in the chamber angle is impaired. If the intraocular pressure rises above the tolerable pressure for the eye in question, progressive damage occurs to the weakest part, the optic nerve. The increasing optic nerve damage leads to a progressive impairment of the visual field. It is important to emphasize that the optimal tolerable internal pressure is different for each eye; progressive glaucoma can develop even with a normal-low internal pressure. Therefore, current definitions of glaucoma omit internal pressure as the main factor of optic nerve damage and place it on an equal footing with the other risk factors [3]. These risk factors are described in more detail in the section on diagnostics.
Division
Glaucomas can be classified either by their structure or by their etiology. Structurally, we distinguish the following forms:
- Open angle glaucomas (approximately 90%) show a consistently open chamber angle. The aqueous humor outflow obstruction is mainly located at the level of the trabecular meshwork and at the junction with the episcleral veins and leads to a more or less pronounced increase in intraocular pressure. A subgroup are patients with a so-called normal pressure glaucoma, in which the intraocular pressure is always in the normal range, but the optic nerve shows signs of increasing damage.
- Angle block glaucomas (about 5%) show a more or less closed chamber angle. Patients with a so-called narrow-angle situation must be informed about the findings, since a rapid increase in eye pressure may occur under certain conditions. This rapid increase of eye pressure is an ophthalmologic emergency situation: The acute glaucoma attack is very painful and leads to ocular symptoms as well as to a pronounced impairment of the general condition.
In a classification according to etiology, we distinguish two forms:
- In primary glaucoma (approximately 95%), glaucoma is the primary disease of the eye.
- In secondary glaucoma (about 5%), another eye disease is present and glaucoma is a secondary disease.
Congenital glaucomas are a subgroup and the majority of them belong to the primary glaucomas.
Diagnostics
The ophthalmologist takes a medical history to determine the risk profile. We distinguish the following groups as risk factors [4]:
- Family risk factors, especially glaucoma in the next of kin (parents, siblings and grandparents)
- Personal risk factors: Age, ethnicity, and gender are given factors. Partially modifiable are vascular risk factors, and in particular (especially nocturnal) systemic hypotension needs to be addressed. Noteworthy are strong blood pressure fluctuations, which can be recorded during a 24-hour blood pressure measurement. Patients with so-called normal-tension glaucoma often show vascular dysregulation signs with cold acra, Raynaud’s symptoms, migraine and sometimes systemic hypotension. The influence of systemic hypertension and diabetes mellitus is still controversial. Medication history is important: anticholinergics can cause acute glaucoma in patients with a narrow angle situation. Preparations containing cortisone (also in ointments or injection form) lead to an increase in intraocular pressure in one third of patients. The mechanism is based on an increase of outflow resistance in the ventricular angle, for which several theories are discussed and probably a genetic defect is present (“steroid responder”) [5].
- Ocular risk factors: The most important risk factor is an elevated intraocular pressure. As already mentioned, each eye has its maximum tolerable intraocular pressure. Other risk factors include higher refractive anomalies (hyperopia and myopia), a thin cornea (norm: 500-600 microns), intraocular hemorrhage (especially around the optic disc), and deposits in the eye such as pigment dispersion and pseudoexfoliation, which can block outflow in the chamber angle.
During the examination, the refraction and visual acuity of the eyes are determined in order to obtain indications about the visual performance. The morphology of the eyes is assessed on the slit lamp using approximately 10-16x magnification to determine any risk factors. Eye pressure is usually measured by Goldmann applanation tonometry (GAT), which is still the gold standard [6].
Optimal intraocular pressure ranges from 8-21 mm Hg. This is a statistical value, based on an average value of 15 mm Hg and the usual standard deviation.
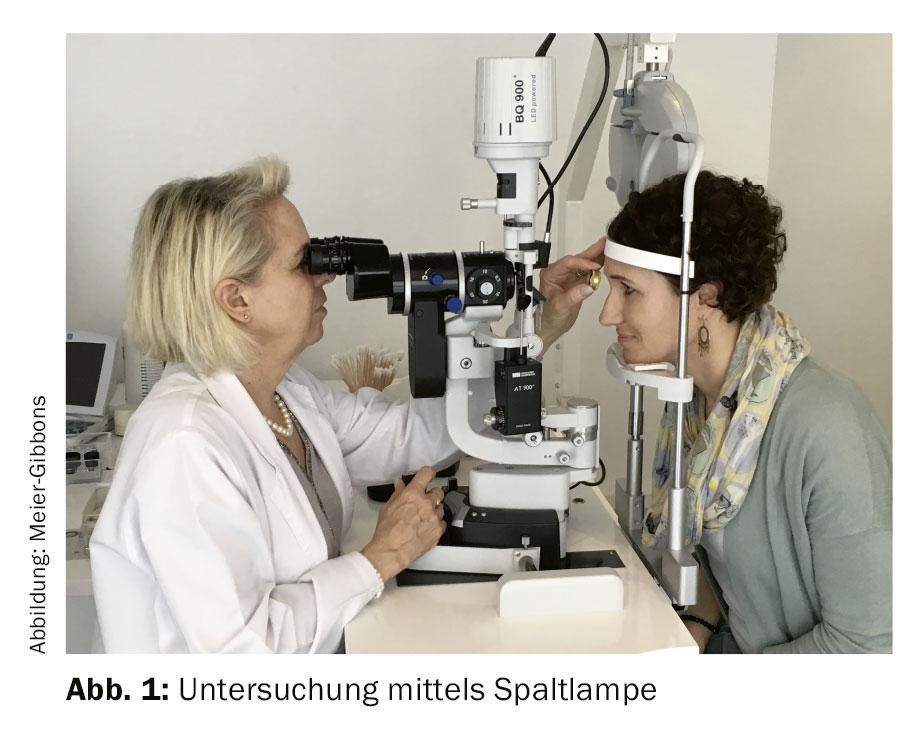
If glaucoma is suspected, additional examinations are ordered (Fig. 1):
- The determination of the visual field
- The morphological representation of the optic nerve
- The representation of the chamber angle by means of a contact glass
- Eye pressure measurements using various measuring devices and determination of corneal thickness.
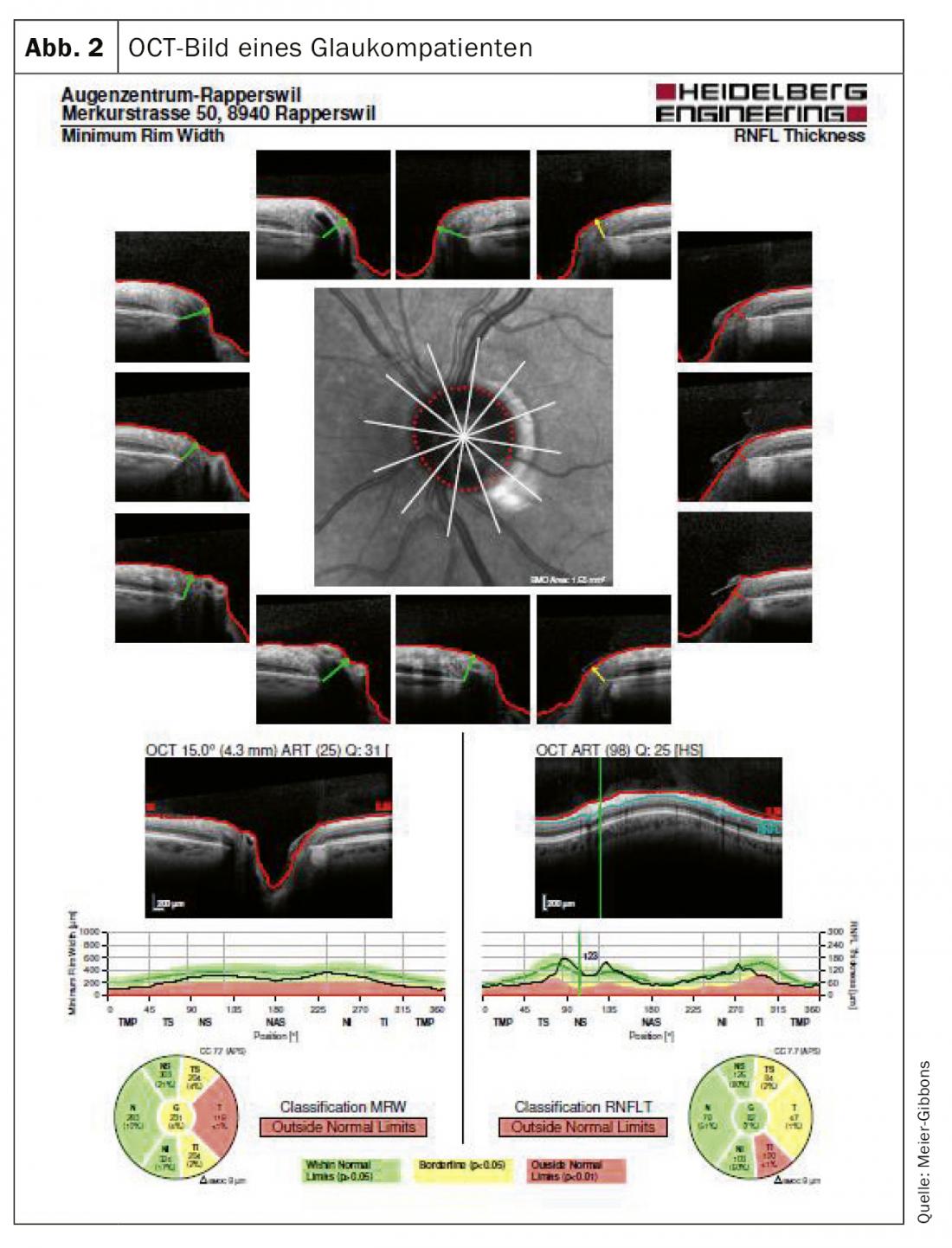
The visual field is determined by means of automatic perimetry. In this examination, the patient is presented monocularly with light stimuli of various sizes and intensities within the normal visual field. The optic nerve should be measured three-dimensionally, for which laser-guided ocular coherence tomography (OCT) is mostly used today (Fig. 2). With this examination, nerve tissue layers can be accurately visualized and compared with a normalized database. Often color photographs are still taken, as these can be used for comparison even after many years, which is unfortunately often not possible with the frequently changing OCT devices (Fig. 3).

It is important to examine the chamber angle using a contact glass. The morphology of the chamber angle provides information about the type of glaucoma present and is important for determining therapy. The examination using contact glass is non-invasive and painless for the patient.
In recent years, several newer eye pressure measurement devices have been developed because studies had shown that measurement according to GAT did not reveal the true intraocular pressure in certain patients (especially in very thin corneas, in the presence of pathological corneal changes, or after corrective treatment by laser). Both these measuring devices and the determination of corneal thickness are used as a supplement to the measurement according to GAT.
All the above mentioned examinations are indispensable for the diagnosis. We distinguish between a normal finding, a finding suspicious of glaucoma and a clearly pathological finding.
It is important to inform the patient accurately: If the eye findings are normal, a check-up is recommended and the patient is informed that the eye may change in the course of life and that certain diseases may only appear at an older age. It is imperative to provide the patient with certain guidelines for the control distance. This depends on age, medical history, local findings and risk factors.
In the case of a finding suspicious for glaucoma, it is imperative to inform the patient: a finding that is borderline at the moment may turn out to be glaucoma in a short time. By definition (American Academy of Ophthalmology and European Glaucoma Society), a patient suspected of having glaucoma has one or more of the following findings:
- The optic disc is suspicious for glaucoma
- The visual field is suspicious for glaucoma
- Eye pressure is higher than 21 mm Hg (it is important to measure eye pressure at different times of the day to determine the highest values)
General remark: Any abnormal examination must first be repeated for safety. If the recurrence really shows pathology, there may be a transition to glaucoma disease.
Therapy
We must be aware that the patient hardly realizes the glaucoma disease, at least at the beginning, and that every therapy has both an effect and often side effects.
The guidelines of the European Glaucoma Society state that any therapy must fulfill the following three main points: it must be both effective and inexpensive, and it must cause as few side effects as possible [3].
At the moment, lowering intraocular pressure is the only therapy that can reduce progression of the disease. Of course, the other risk factors mentioned above must be checked and, if possible, reduced. The influence and protection of the optic nerve itself, the so-called neuroprotection, would be interesting. Unfortunately, previous drug trials have not been very promising.
What options do we have to lower intraocular pressure? There are really only two options:
- Reduction of aqueous humor production
- Improvement of the aqueous humor outflow
Reduction of aqueous humor production: aqueous humor is produced in the ciliary body, circulates in the posterior and anterior chambers of the eye, and exits the eye through the chamber angle into the vascular circulation. The production of aqueous humor can be reduced by beta blockers (BB), alpha agonists (AA), and carbonic anhydrase inhibitors (CAI). The beta-blocker timolol has been used in glaucoma therapy since 1978 and reduces intraocular pressure by 20-25%. Locally, BB are well tolerated, but they often lead to systemic side effects such as lowering of blood pressure and pulse. In our opinion, patients with planned BB therapy should seek discussion with their primary care physician to avoid interference with other medications.
Alpha agonists have been used for many years, although the previously available AA often resulted in very pronounced local and systemic side effects and are therefore no longer used. Today, the most commonly used is brimonidine, which reduces intraocular pressure by 20-25% but can also cause local (hyperemia) and systemic (vascular and cerebral) side effects.
Carboanhydrase inhibitors have been commonly used in glaucoma therapy in systemic form since the early 1950s and have also been used locally since 1994. They sometimes lead to serious systemic side effects, especially in the presence of sulfonamide hypersensitivity. Both alpha agonists and carbonic anhydrase inhibitors are sometimes used in combination with beta blockers to achieve better eye pressure reduction.
Improvement of aqueous humor outflow: The aqueous humor leaves the eye in two ways: The conventional or trabecular outflow and much less frequently the unconventional or uveoscleral outflow. The latter corresponds to a proportion of 25-55% among children and decreases progressively with age. A decrease in aqueous humor outflow leads to backwater in the eye and subsequently to an increasing increase in intraocular pressure. Recent studies have shown that the majority of the crucial increase in outflow resistance is in the region of the iuxtacanalicular trabecular meshwork and in the region of the inner wall of Schlemm’s canal.
The main drugs used to improve aqueous humor outflow are the local prostaglandin agonists. They were first used in glaucoma therapy in the mid-1990s and after a very short time took over the leading position of antiglaucomatous drugs. The first product was latanoprost, followed by several similar drugs. The effect (an approximately 25% reduction in eye pressure) lasts 24 hours, so patient adherence is better. The prostaglandin agonists show practically no systemic side effects, but the local side effects could be considerable: Eyelash growth, increasing iris pigmentation, hyperemia of the conjunctiva, but also darker periorbital skin discoloration and atrophy of orbital adipose tissue.
In some countries (not yet in Europe) two new substance groups are approved in glaucoma therapy: The rhokinase inhibitors and the latanoprost Bunod. Both drugs improve aqueous humor outflow and also need to be used only once a day.
It is important for us to briefly address the issue of generic drugs, which are being used more and more frequently. By definition, the content of generic drugs should be identical to the originator drugs. However, eye drops differ from systemically applied medications in important ways:
- We can determine the concentration of the active substance in the eye drop, but the bioavailability of the substance on the eye itself cannot be measured.
- Only the active ingredient of the generic drug must be identical to the originator drug; all excipients may vary. Since the active ingredient in latanoprost, for example, is only 0.005%, most of the drop may be altered in a generic drug.
- Many studies showed differences between originator and generic drugs in terms of size and viscosity of the drop, nature of the drop bottle, opening of the drop bottle, and preservative [7].
Therefore, in our opinion, switching to a generic drug is equivalent to using a new drug and requires more frequent controls at the beginning.
Problematic is the frequent switch to cheaper and cheaper generics by pharmacies, which can lead to neither the patient nor the doctor knowing which drug they are using at the moment. A discussion with the patient is worthwhile to point out the differences between originator and generic products.
It is not easy to find an antiglaucomatous therapy that reduces disease progression, has few side effects, and is used regularly. Adherence and persistence are not very good in glaucoma, as in other chronic diseases. Many studies have shown that adherence to prescribed therapy is 30-70% and that after one year only 10% of patients reorder the prescribed eye drops [8].
The reasons for poor adherence are many, but we must be aware that we are prescribing a therapy that must be used extremely reliably and regularly, but also frequently has side effects. This is especially problematic in patients in early stages of glaucoma, as they are still hardly aware of the disease itself. Newman Casey summarized the most common reasons for lack of adherence in a study: Difficulty with drop application, difficulty integrating drop application into daily routine, and side effects of drops [9]. The side effects should not be underestimated. Studies have shown that two-thirds of glaucoma patients suffer side effects from their therapy [10]. It is both local side effects of the drugs and late effects on the eye and especially on the ocular surface that bother the patient. If a patient shows side effects of his therapy, a change of therapy is worthwhile, possibly also from one prostaglandin derivative to another derivative. In younger patients, patients on multiple medications, and patients with pre-existing ocular surface disorders (“sicca syndrome”), it is worthwhile to try preservative-free eye drops. Preservatives, especially benzalkonium chloride, can lead to an increase in ocular surface problems. However, because the preservative-free eye drops are often packaged in single doses, patients with poor visual acuity or rheumatic changes in their hands may have difficulty applying them [11].
In addition to antiglaucomatous medications, eye pressure can be lowered by surgical means. The first surgical procedure for glaucoma was performed in 1856 (iridectomy by Von Graefe), and recently the surgical spectrum has expanded significantly with the introduction of microinvasive glaucoma surgery (MIGS). Less invasive are various laser techniques that can both decrease aqueous humor production and improve aqueous humor outflow.
Surgery is considered especially in young patients with advanced glaucoma or patients with intolerance of local therapy (mostly ocular surface problems).
Outlook for the future
The fact that still many patients go blind from glaucoma is frightening, because the disease, if caught in time and treated properly, can be clearly influenced in progression. What do we need to work on?
There are still at least 50% of patients who do not know that they have this disease. Therefore, accurate education of the population is mandatory to detect those who suffer from rapidly proged glaucoma by evaluating the patients and their risk factors.
If a glaucoma patient starts a therapy, the aspect of adherence has to be discussed with the patient: A drug applied in drop form is only effective if the drop is applied!
Regular checks of the optic nerve and its function are mandatory; the eye pressure must be controlled and the therapy adjusted accordingly. A patient diagnosed with glaucoma is usually checked by an ophthalmologist 2-3× per year. Newer drugs with fewer side effects and possibly different mode of application (for example, punctum plugs with slow-release form or by injection into the eye itself) may lead to better adherence and thus better management of glaucoma.
The alternatives to drug therapy are important: Laser therapy can improve the outflow of aqueous humor; in addition, surgical therapy shows many new approaches, so-called “microinvasive” interventions are increasingly performed, in which stents are sometimes inserted.
The patient himself is and will continue to be important: he must be precisely informed about the disease and involved in the therapy of his disease.
Take-Home Messages
- Despite improved diagnostic and therapeutic measures, glaucoma still leads to reduced quality of life and sometimes blindness in many patients. The number of patients who are unaware of their disease is 50% even in industrialized countries and requires increased public information about the disease.
- The physician should take a detailed history in all adults to assess the risk factors for developing glaucoma (familial, systemic, and ocular). From the age of 40, a preventive examination by an ophthalmologist is necessary. The so-called “preventive examinations”, which are sometimes performed by opticians, are in no way sufficient.
- The only therapy for glaucoma so far is the treatment of the most important risk factor, the elevated eye pressure. This therapy is mostly done with local medications that lower the eye pressure; with the increase of surgical options, direct surgical therapy will probably be used more frequently in the future.
- A good cooperation between the treating ophthalmologist and the general practitioner is important for all therapeutic measures.
Literature:
- Quigley HA, West SK, Rodriguez J, et al: The prevalence of glaucoma in a populationbased study of Hispanic subjects: Proyecto VER. Arch Ophthalmol 2001; 119: 1819-1826.
- Bulletin of the World Health Organization, Nov 2004; 82 (11).
- EGS Guidelines Edition 2014.
- Leske CM et al. For the Early Manifest Glaucoma Trial Group. Arch Ophthalmol. 2003;121(1): 48-56.
- Kersey JP, Broadway DC: Corticosteroid-induced glaucoma: a review of the literature. Eye 2006; 20: 407-416.
- Goldmann H, Schmidt T: On applanation tonometry. Ophthalmologica 1957; 134: 221-242.
- Genazzani AA, Pattarino F: Difficulties in the production of identical drug productfrom a pharmaceutical technology viewpoint. Drugs RD 2008; 9(2): 65-72.
- Friedman DS, Quigley HA et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007 Nov; 48(11): 5052-5057.
- Newman-Casey PA, Killeen OJ, Renner M, et al: Access to and experiences with e-health technology among glaucoma patients and their relationship with medication adherence. Telemed J E Health 2018; DOI: 10.1089/tmj.2017.0324.
- Zimmerman JB, Hahn SR, Gelb L, et al: The impact of ocular adverse effects in patients treated with topical prostaglandin analogs: changes in prescription patterns and patient persistence. J Ocul Pharmacol Ther: 1308-1316.
- Dietlein TS, Jordan JF, Lüke C, et al: Self-application of single-use eyedrop containers in an elderly population: comparisons with standard eyedrop bottle and with younger patients. Acta Ophthalmol. 2008; 86: 856-859.
FAMILY PRACTICE 2019; 14(11): 6-10