Diabetic polyneuropathy (dPNP) is the most common neuropathy in Western countries. But who gets them and how do you recognize them? This question is particularly important because 40% of those patients whose dPNP is painful still go untreated. Possibilities of diagnostics and therapy were the topic at the German Pain Congress.
Somatosensory dPNP almost always develops in parallel with retinopathy or nephropathy. Therefore, one should always keep an eye on the eyes and kidneys during diagnostics, explained Prof. Dr. Frank Birklein from the Clinic and Polyclinic for Neurology at the University Hospital Mainz (D) by way of introduction. The only exception to this is small fiber neuropathy (SFN), which may precede diabetes. 50% of diabetics get PNP electrophysiologically, and in 16% of these sufferers the PNP is painful. One quarter of all diabetics clinically have PNP. 40% of painful dPNP are untreated.
Pathophysiologically, there are alterations in metabolism in dPNP, especially in diabetes metabolism. Important here is the polyol pathway and the advanced glycation endproducts (AGE), which accumulate and damage neurons and especially axons. In addition, there are changes in ion channels, especially potassium and sodium channels, and mitochondrial defects appear, which ultimately lead to neurons getting too little energy. It should not be forgotten that structural changes also occur, especially in the vessels. Vascularly, this is characterized by the fact that veins and arteries are morphologically inconspicuous in control neuropathies, whereas in diabetics the artery is reduced in size and “coils up”, thus there is an arteriolopathy, the veins are partially congested. A diabetic nerve ultimately has too little oxygen and is hypoxic.
Diagnostics
The first step in the diagnosis should be the measurement of nerve conduction velocities (NLG) – either performed by oneself or by a trusted neurological colleague, as Prof. Birklein put it: “I always believe normal values from a practice, pathological ones, on the other hand, are not always correct, because the patient has not been properly warmed up, for example. Cold extremities lead to a slowing of nerve conduction velocity.” The program of NLG measurement includes two nerves motor and sensitive at the upper extremity. Prof. Birklein recommends at least the ulnar, as the median often has carpal tunnel syndrome (CTS) and misdiagnosis can occur if one is not aware of it. “And diabetic patients now very often have CTS.” On the lower extremities, two motor nerves (tibialis, peroneus) and the suralis should be sensitively addressed. The expert recalled that there is little movement in a diabetic’s nerve conduction velocity over a period of up to five years, and it is usually at a consistently reduced level. The situation is different for the amplitudes of the motor sum action potentials, which can be stimulated, for example, when measuring the peroneus. here it becomes less and less over the course of 2 years. This means that the axons break down during dPNP. “This is not a neuronopathy, it’s a dying back axonopathy,” the neurologist explained. The longest nerve fibers are affected first and the axons die back. As a result, the amplitude becomes smaller and smaller.
If the NLG is normal, the patient does not have PNP. Usually, the neurological findings are then also normal, but the affected person still has burning pain (sfPNP in the early stage). In this case, it is quite sufficient to determine the cold and warm thresholds according to the QST* protocol to diagnose sfPNP. The physician erodes the loss of the sensation of cold and warm. Cold corresponds to delta function, so fast-conducting nociceptors are the same fiber class, and warm is C class, slow-conducting nociceptors. The cold threshold of healthy subjects at the foot is starting from 32°C – a reduction of 4°C is felt by a healthy subject, while in patients this is only the case from 12°C onwards. At the warm threshold, i.e. measured from 32°C after warmer temperatures, healthy people need about 6°C difference at the foot to feel anything, patients with PNP or diabetes accordingly more. This also remains constant over two years with a slight increase.
(* Quantitative Sensory Testing)
When measurement of warm and cold thresholds is not helpful, the standard of care in the diagnosis of sfPNP is a skin biopsy, which is offered by many neuropathology laboratories.
Prevention
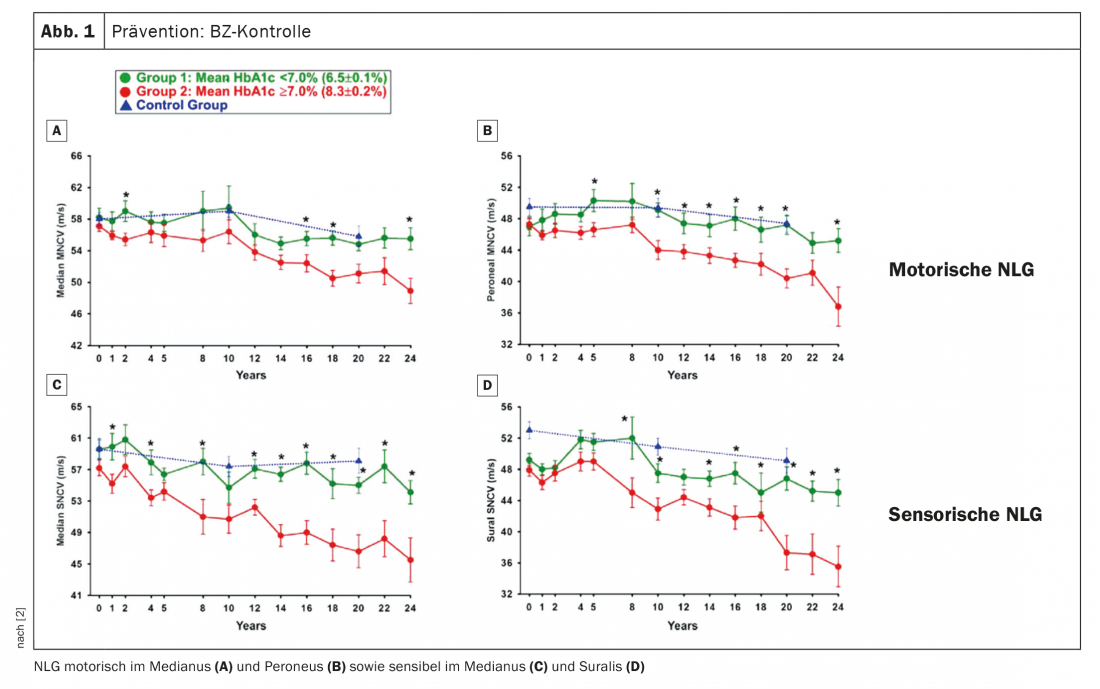
How can the occurrence or progression of dPNP be prevented? First of all, of course, the control of blood glucose (BG) is obvious for this. Prof. Birklein referred to a paper in which nerve conduction velocities were measured over more than 20 years (Fig. 1). One can see in it that the NLG of healthy controls goes down somewhat over the period of two decades, but that is quite normal. Patients with an HbA1c ≥7, i.e., conventional BG adjustment, were compared with patients with intensified BG adjustment. What you see is that patients with intensified BG control in type 1 diabetes have a course that you would expect in a healthy person. “If the diabetes is well controlled, then you will not get PNP for many years” was the expert’s conclusion. On the other hand, if it is worse adjusted, PNP develops. Conclusion: BG control is essential, especially in young people who develop diabetes. Intensified recruitment is also recommended, not least for this reason.
In type 2 diabetes, the situation is somewhat different. Here, it is mainly cardiovascular risk factors that need to be taken into account: Hypertension, smoking, BMI, triglycerides, and of course HbA1c. “People don’t think of the cardiovascular factors in PNP that way, but just think of the vascular changes in the peripheral nerve,” Prof Birklein cautioned.
15% of dPNP patients have pain
But why do 15% of dPNP patients have pain? First, one thing should not be forgotten, the expert said: Not all patients who have diabetes and dPNP must also have neuropathic pain. Patients with diabetes and advanced neuropathy may also develop gonarthrosis or synovitis, for example, which are responsible for the pain. These possibilities should always be kept in mind.
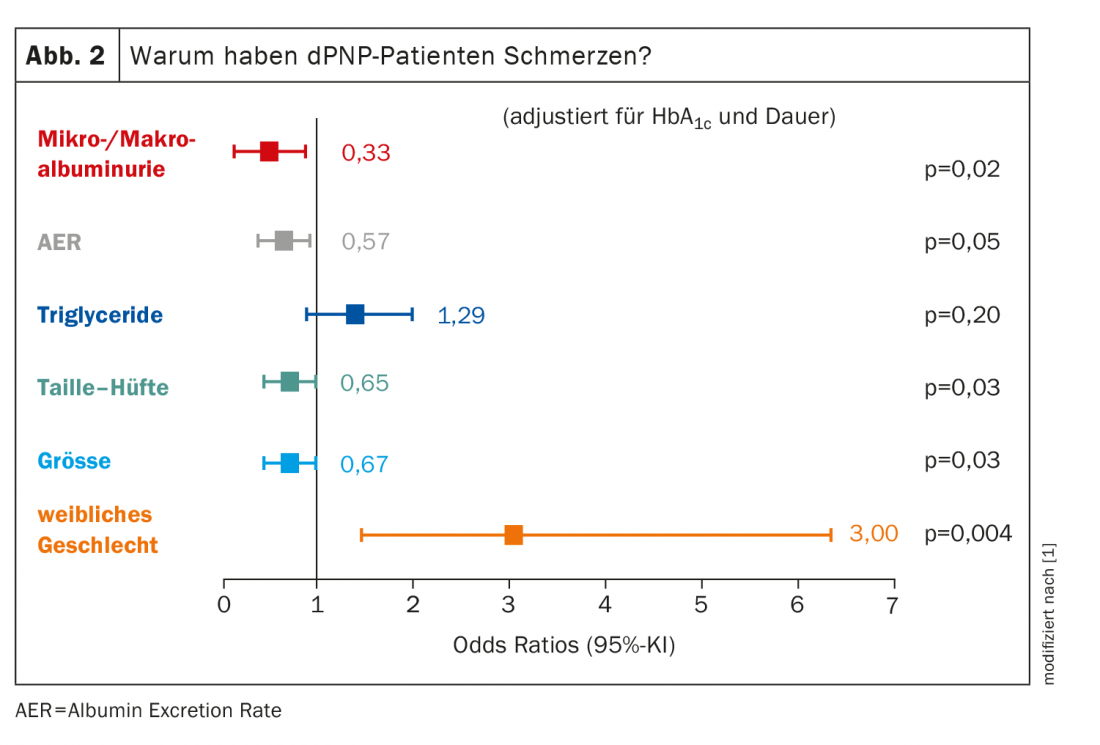
With regard to the group of painful dPNP patients whose cause is really polyneuropathy, it is mainly women who are affected: Female gender has an odds ratio of 3.00 for painful PNP here. All other factors account for significantly less (Fig. 2) . Body size plays a role, which may sound surprising at first. However, the longer the axons, the more likely damage will occur. “We always have to remember: the metabolism of the whole axon is done in the neuron – more specifically, in the posterior root ganglion, as far as the pain fibers are concerned – and it has to be transported all the way to the nerve terminals. And the larger they are, the greater the likelihood that something will go wrong along the way.” The more axons that are damaged, the more painful the dPNP.
So where does neuropathic pain come from? When axon damage occurs, the neuron becomes electrically unstable and spontaneously active, the pain physician explained. This occurs through sodium (Na+) channels. If the sodium channels are blocked, then a damaged neuron that has been axotomized is also completely quiescent again. Activation of sodium channels probably functions via methylglyoxal. Glucose is converted to methylglyoxal in plasma. Glyoxalase – an enzyme that is the rate limiting step here – then causes the methylglyoxal to be converted to lactate. Finally, if glyoxalase is not functioning well, methylglyoxal accumulates. Measurements of methylglyoxal in plasma have shown that patients with diabetes without pain have an increased amount of methylglyoxal – because they naturally have more glucose than healthy people. However, the patients with pain once again have a significantly increased amount of methylglyoxal in their blood compared to the non-pain patients.
Therapy of dPNP
Regarding the treatment of patients with diabetic polyneuropathy, Prof. Birklein listed a number of pharmacological options that are considered first-line agents:
- Anticonvulsants (Ca-channel blockers – gabapentin, pregabalin)
- Tricyclic antidepressants
- Serotonin-norepinephrine reuptake inhibitor (SSNRI) (duloxetine).
- Capsaicin patch
- Lidocaine patch
According to the current S2 guideline, carbamazepine, oxcarbazepine, lamotrigine, venlafaxine, and cannabinoids may also be considered in individual cases. Prof. Birklein is not very convinced of the plaster options, “in some patients you would have to plaster over half the leg, and that’s not very nice. As an alternative, he reminded the audience of the non-drug options available – knowing full well that many sufferers ultimately prefer to swallow pills rather than exercise. If neither medication nor exercise brings the desired success, there is also spinal cord electrostimulation (SCS), which has worked well in studies of pain-stricken PNP patients.
– German Pain Congress 2020, Mannheim (D) and online
Sources:
- Diabetic polyneuropathy: From Bench to Bedside. New data on pathophysiology, diagnosis, and therapy. German Pain Congress Mannheim, 22.10.2020
- Ziegler D, et al: BMJ Open 2015; 5: e006559; doi: 10.1136/bmjopen-2014-006559
InFo PAIN & GERIATRY 2020; 2(2): 24-25 (published 9/12/20, ahead of print).