The episodic scaling dermatosis leads to a high degree of suffering. Dermatological shampoos containing antifungal substances have been shown to alleviate symptoms in the capillitium area. In a multicenter study, miconazole nitrate 2% and ketoconazole 2% for the treatment of seborrheic dermatitis of the scalp were found to be comparable in terms of both efficacy and tolerability.
Seborrheic eczema is a chronic recurrent inflammatory skin disease characterized by scaling, itching and erythematous plaques. Typical predilection sites include the capillitium and face [1–3]. The etiopathogenesis is not yet fully understood; a multifactorial structure is assumed. There is evidence that excessive colonization of the scalp with the yeast Malassezia furfur (Pityrosporum ovale) due to increased production of the sebaceous glands plays an important role [2,4,5]. Antifungal therapy leading to a reduction in Malassezia colonization on the scalp has been shown to be an effective method of therapy, azole antifungals in particular have been shown to be effective [3,6].
Comparative study of topical azole-containing antifungal agents.
The aim of a study published in the Journal of Dermatological Treatment by Buechner et al. consisted in demonstrating the non-inferiority of miconazole nitrate compared to ketoconazole in the topical treatment of seborrheic dermatitis on the scalp [8]. In the randomized-double-blind multicenter comparative study in a parallel-group design, miconazole nitrate 2% shampoo* vs. ketoconazole 2% shampoo were each applied at a dosage of 10 ml, 2× weekly, for a treatment period of 4 weeks (n=145, n=129). The severity of the leading symptoms (erythema, scaling and itching) was operationalized using the “Symptom Scale of Seborrheic Dermatitis” (SSSD). The age range of the subjects was 18-70 years, and the inclusion criteria included a baseline score of at least 6 points on the SSSD scale.
* Content composition of the shampoo containing miconazole nitrate: 2% miconazole nitrate, 0.75% piroctonolamine, 8% urea.
Primary and secondary efficacy endpoints met
The demographic characteristics of the two treatment groups at baseline were similar, including mean age (miconazole group: 42.0 years, ketoconazole group: 42.3 years) [8]. Disease severity at baseline also did not differ significantly between the two treatment groups (mean SSSD score 8.3 vs. 8.1).
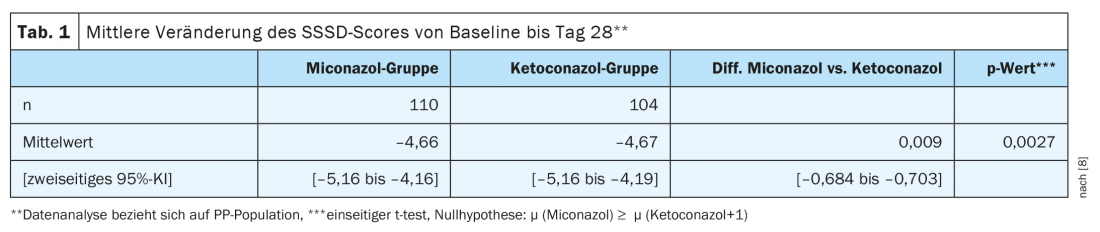
The primary endpoint of the study was the reduction in the Symptom Scale of Seborrheic Dermatitis (SSSD) after a treatment period of 4 weeks. To evaluate this efficacy endpoint, individual change in SSSD score was measured from baseline (day 0, assessment 1) to day 28 (assessment 3). Due to the “non-inferiority” design, data analysis was performed in the PP** study population. The null hypothesis of >1 inferiority in the SSSD score of treatment with miconazole compared with ketoconazole was rejected, with a predefined type 1 error rate of 0.025 in the one-sided t test (Table 1) . The treatment effects of the two study arms did not differ significantly. The adjusted difference between the miconazole and ketoconazole groups was 0.21 SSSD score points (95% CI: 0.42-0.85).
** PP study population= “Per-Protocol” study population.
Conclusion
Overall, in the present comparative study, all of the interference statistical analyses show non-inferiority of the miconazole-containing treatment compared with the ketoconazole formulation. Secondary efficacy endpoints, including subscores in the SSSD and CGI*** and proportion of responders$ also proved comparable in both treatment groups, underscoring the equivalence of the two treatment options. Both miconazole and ketoconazole were convincingly well tolerated. Most of the drug-related side effects were mild to moderate and limited to mild burning or itching of the scalp.
*** CGI = Clinical Global Impression
$
Responders were defined as subjects who achieved an SSSD score of at least 3.
Literature:
- Aschoff R, Kempter W, Meurer M. [Seborrheic dermatitis]. Dermatologist 2011; 62: 297-307.
- Hay RJ. Malassezia, dandruff and seborrheic dermatitis: an overview. Br J Dermatol 2011; 165: 2-8.
- Naldi L, Rebora A. Clinical practice. Seborrheic dermatitis. N Engl J Med. 2009; 360: 387-396.
- DeAngelis YM, et al: Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J Investig Dermatol Symp Proc 2005; 10: 295-297.
- Tajima M, et al: Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: comparison with other diseases and healthy subjects. J Invest Dermatol 2008; 128: 345-351.
- Gupta AK, Nicol K, Batra R: Role of antifungal agents in the treatment of seborrheic dermatitis. Am J Clin Dermatol 2004; 5: 417-422.
- Quatresooz P, et al: Novelties in the multifaceted miconazole effects on skin disorders. Expert Opin Pharmacother 2008; 9: 1927-1934.
- Buechner SA: Multicenter, double-blind, parallel group study investigating the non-inferiority of efficacy and safety of a 2% miconazole nitrate shampoo in comparison with a 2% ketoconazole shampoo in the treatment of seborrheic dermatitis of the scalp. Journal of Dermatological Treatment 2013; Early online 1-6.
DERMATOLOGY PRACTICE 2021; 31(4): 30











