The use of percutaneous mitral valve repair using a MitraClip device for the treatment of (moderately) severe functional mitral regurgitation is controversial. Previous studies were either terminated due to insufficient recruitment or were negative. With the COAPT study, a positive result regarding hospitalization frequency and secondary endpoints (incl. all-cause mortality) is available for the first time. A discussion of previous study findings.
Cardiology has changed dramatically in recent decades with major advances in the understanding and treatment of heart disease. Accordingly, it is now difficult for general practitioners to care for patients with heart disease on their own. Heart failure, which is comparable to malignant tumors in terms of mortality and morbidity [1], requires specialized diagnosis and therapy [2].
Status and epidemiology of heart failure.
Heart failure is a symptom complex, i.e. a syndrome and sequelae of various cardiac diseases. Despite all protestations, it is unfortunately also too often insufficiently clarified and treated in Switzerland. The involvement of a specialist, namely a cardiologist, is an absolute necessity.
The European Society of Cardiology (ESC) has addressed this development by establishing comprehensive guidelines that define evidence-based practice not only for interventional cardiology, rhythmology, and heart failure, but for many other areas of cardiology.
Heart failure is epidemiologically relevant. There are now approximately 200,000 heart failure patients in Switzerland, with approximately 10,000 new cases annually [2,3]. Heart failure is the most common reason for hospitalization among >65-year-olds. Increasing aging and better treatment options for acute cardiac conditions represent the most important causes of the increasing prevalence and incidence of heart failure.
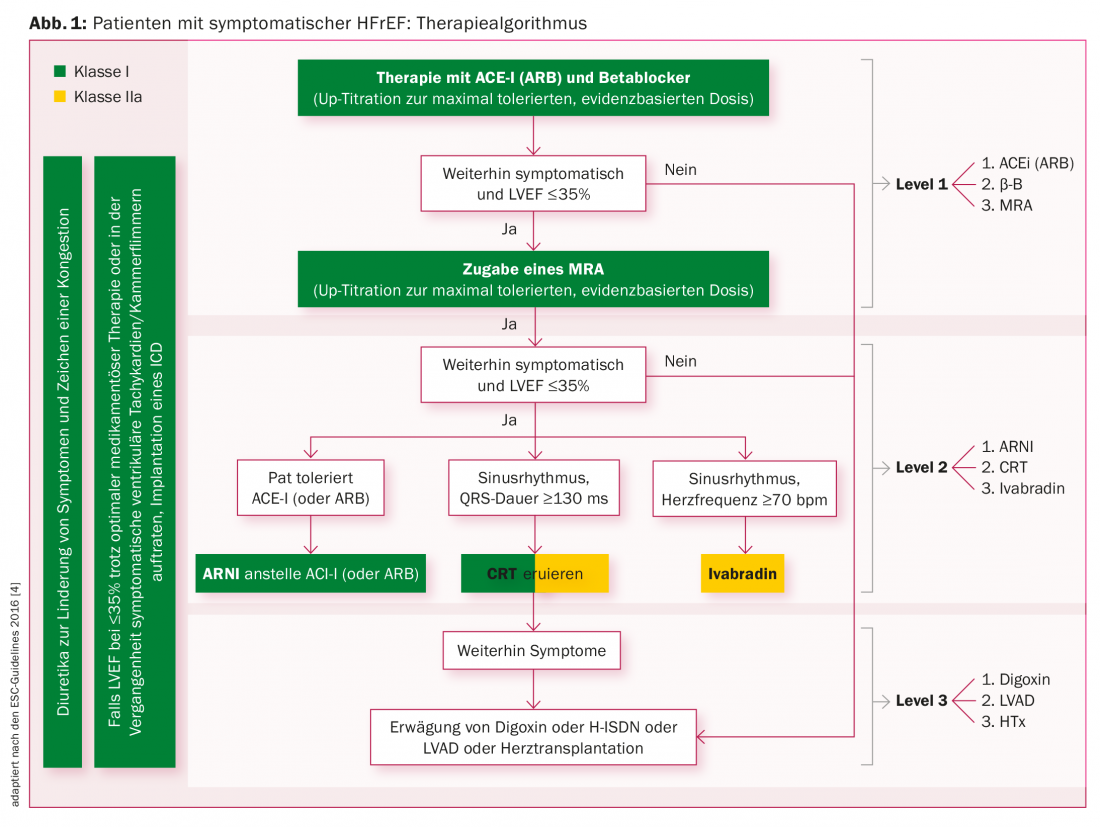
Treatment algorithms
Another fact is the inadequate, target-dose-oriented drug therapy with ACE inhibitors (resp. Angiotensin receptor blockers [ARB]), beta blockers, and aldosterone inhibitors (mineralocorticoid receptor antagonists, MRA) [4]. An overview of the treatment algorithm for patients with symptomatic HFrEF is given in Figure 1 . If patients are still symptomatic despite extended therapy with “level 1” drugs, “level 2” measures should be considered or used. Valsartan/sacubitril (Entresto®) is currently used as a “Level 2” medication according to the PARADIGM study [5]. The same applies to the use of ivabradine (Procoralan®) and cardiac resynchronization.
Heart failure and percutaneous mitral valve reconstruction
The value of a new treatment concept is unclear: percutaneous mitral valve reconstruction using MitraClip devices. It should be used in heart failure patients with functional moderate to severe mitral regurgitation (at least class 3 [6]). Until a few days ago, the MitraClip was not approved in heart failure patients with functional mitral regurgitation in the USA (waiting for the COAPT trial, see below and [7]) and had a IIb indication (evidence level C) in Europe: The RESHAPE trial was interrupted because the recruitment rate was smaller than originally predicted. As recently as May 2015, the RESHAPE-HF1-FU study had a goal of. However, on the official website, the last update was in August 2016; the recruitment status is unknown [8].
The MITRA-FR study presented at the ESC Congress in August 2018 was negative in 304 patients [9]. There were 452 patients recruited and 145 excluded during the screening process. 109 patients were ultimately in the intervention group and 137 patients were in the control group. Thirty-seven study centers in France participated, resulting in an average of 6.6 patients at each center. Symptomatic (NYHA II to IV) heart failure patients with secondary mitral regurgitation (defined as “effective regurgitant orifice area (ERO)” of >20 mm2 or regurgitant volume of >30 ml per beat) and LVEF of 15-40% were included. The recruitment phase ran between December 2013 and March 2017. Centers had to have experience with percutaneous interventions and at least five Abbott Vascular study MitraClips previously implanted. The combined primary end point (at 12 months) was mortality from any cause or unplanned hospitalization for heart failure. Secondary end points were left ventricular EF, LVESD and LVEDD, LV volume, severity of mitral regurgitation postintervention, NYHA class, 6-minute walk test (6-MWT), BNP, and EQ-5D (“European Quality of Life 5-Dimension Scale”).
| “Even a parachute doesn’t work if you open it too late.” Francesco Maisano (Zurich), CRT Congress 2018 |
Demographic and clinical characteristics were similar in the intervention and control groups, except that a previous myocardial infarction was more common in the intervention group. The mean age was seventy years. NYHA class II could be attributed to 36.8% of the intervention group vs. 28.9% of the control group, and NYHA class III to 53.9% vs. 63.2%. LVEF was 33.3% versus 32.9% in the intervention group. The ERO was 31 mm2 in both groups, and the regurgitation volume was 45 ml in each. BNP was 765 ng/l vs. 835 ng/l. The periprocedural complication rate was relatively high at 14.6%. The primary and secondary endpoints were not statistically different (primary endpoint: p=0.53).
In summary, this is a relatively small prospective controlled randomized trial (CRT) with, it can be assumed, relatively small and relatively inexperienced centers.
Mitral valve intervention: back on track for functional mitral regurgitation
The COAPT study, also sponsored by the industry involved (Abbott Vascular), was presented just a few days ago at TCT 2018. It shows for the first time a statistically significant and beneficial effect on repeat hospitalization frequency due to heart failure and a low complication rate due to the MitraClip in heart failure patients with functional mitral regurgitation [7]. All-cause mortality was also reduced by the MitraClip, which was actually a secondary endpoint.
In the COAPT study, 614 patients were recruited. The intervention group consisted of 302 patients, and the control group consisted of 312 patients. A total of 78 study sites in the U.S. and Canada participated. Symptomatic (NYHA II to IV) heart failure patients with secondary mitral regurgitation (defined as moderate [Grad 3+] to severe [Grad 4+]) and LVEF of 20-50% were included. The recruitment phase ran between December 2012 and June 2017. The primary end point (at 24 months) was unplanned hospitalization for heart failure and absence of a MitraClip-related complication at 12 months. Secondary end points were the degree of mitral regurgitation and death from any cause at 12 months. Combined end points related to death or hospitalization for heart failure at 24 months, quality of life (KCCQ), the 6-MWT, hospitalization frequency from any cause, NYHA class, or death from any cause at 24 months.
Demographic and clinical characteristics were similar in the intervention and control groups. The mean age was 71.7 years in the intervention group and 72.8 years in the control group. 42.7% of the intervention group corresponded to NYHA class II, compared with 35.4% in the control group. NYHA class III heart failure was present in 51% (intervention group) vs. 54% (control group). LVEF was 31.3% vs. 31.3%, ERO was 41 mm2 vs. 40 mm2, regurgitation volume was 59 ml vs. 57 ml, and BNP was 1014 ng/l vs. 1017 ng/l. The complication rate at 12 months was low at 5.2%. The outcome regarding the primary endpoint hospitalization for heart failure reached statistical significance in favor of the intervention group (p<0.001). All secondary endpoints were also statistically significantly better in favor of the intervention group.
In summary, this is a first-time CRT with a larger patient population compared with the MITRA-FR trial. Although recently renowned experts expressed their fear that the patients of the COAPT study might be treated with a MitraClip “too late” (“even a parachute doesn’t work if you open it too late”), the patients of the COAPT study seem to have a more pronounced mitral regurgitation compared to the MITRA-FR study (see e.g. BNP, ERO area, regurgitation volume). Clinically, however, the COAPT patients seemed rather better than the MITRA-FR trial patients in terms of their heart failure (see, for example, LV end-diastolic volumes 101 ml/m2 vs. 135 ml/m2). Moreover, the involved COAPT centers from the USA and Canada are renowned centers (see low complication rate).
It remains to be seen how the COAPT study will be classified. For the time being, the clinical experience of heart failure cardiologists has not yet been able to confirm these very positive results, but one should be open to new developments, knowing full well that industry pressure is not negligible at times. However, it appears that the COAPT study was seriously designed and conducted. We are looking forward to the details of the study, the discussion within the concerned professional societies, but especially to the RESHAPE-HF1-FU study.
Take-Home Messages
- Heart failure is a common and malignant disease, which – similar to a tumor in oncology – must be clarified by the cardiologist. Evidence-based heart failure treatment includes an algorithm of measures with appropriate target doses.
- The use of percutaneous mitral valve repair using a MitraClip device for the treatment of (moderately) severe functional mitral regurgitation is controversial.
- Previous prospective controlled randomized trials regarding MitraClip device for the treatment of functional (moderate)severe
- Mitral regurgitation were either discontinued because of insufficient recruitment or were negative. With the COAPT study, a positive result regarding hospitalization frequency and secondary endpoints (incl. all-cause mortality) is available for the first time. Where this study should be classified, however, remains to be seen. The COAPT study appears to have been conducted in a reputable manner.
Literature:
- Stewart S, et al: More ‘malignant’ than cancer? Five-year survival following first admission for heart failure. Europ J Heart Fail 2001; 3: 315-322.
- Mohacsi P, et al: Position Paper “Heart Failure-.
- Curriculum” of the Heart Failure Working Group of the SGK. Cardiovascular Medicine 2018; 21: 26-32.
- Moschovitis G, et al: The Swiss Heart Failure Registry: a longitudinal follow-up survey. Cardivascular Medicine 2002; 5: 15.
- Ponikowski P, et al: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Europ Heart J 2016; 37: 2129-2200.
- McMurray JJV, et al: Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993-1004.
- www.csecho.ca/wp-content/themes/twentyeleven-csecho/cardiomath/?eqnHD=echo&eqnDisp=pisamr. Accessed 9/26/2018.
- Stone GW, et al: Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018; DOI: 10.1056/NEJMoa1806640.
- The RESHAPE-HF1-FU Study: https://clinicaltrials.gov/ct2/show/NCT02444286. Accessed 9/26/2018.
- Obadia JF, et al: Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; DOI: 10.1056/NEJMoa1805374.
CARDIOVASC 2018; 17(5): 10-13











