Diabetes is the most common cause of chronic kidney disease (CKD).
The international organization Kidney Disease: Improving Global Outcomes (KDIGO) recently released new guidelines on the management of type 2 diabetes in people with CKD. A treatment approach is propagated that is based on several pillars. One of the key messages: the progression of kidney disease can be slowed with new drugs, sparing many from dialysis for a long time.
Whereas previously there were few therapeutic options available that could halt the progression of diabetes-associated CKD, there are currently several modern classes of drugs that can significantly slow renal function loss if used in a timely manner. Therefore, it is important that affected individuals receive timely co-management by a nephrologist [1]. “Criteria for presenting the person with diabetes mellitus to the nephrologist are, for example: new or significant eGFR drop, eGFR ≤60 ml/min/1.73 m², erythrocyturia, or grade 2 or 3 albuminuria,” explained Prof. Julia Weinmann-Menke, MD, Mainz, press officer of the German Society of Nephrology [1,3].
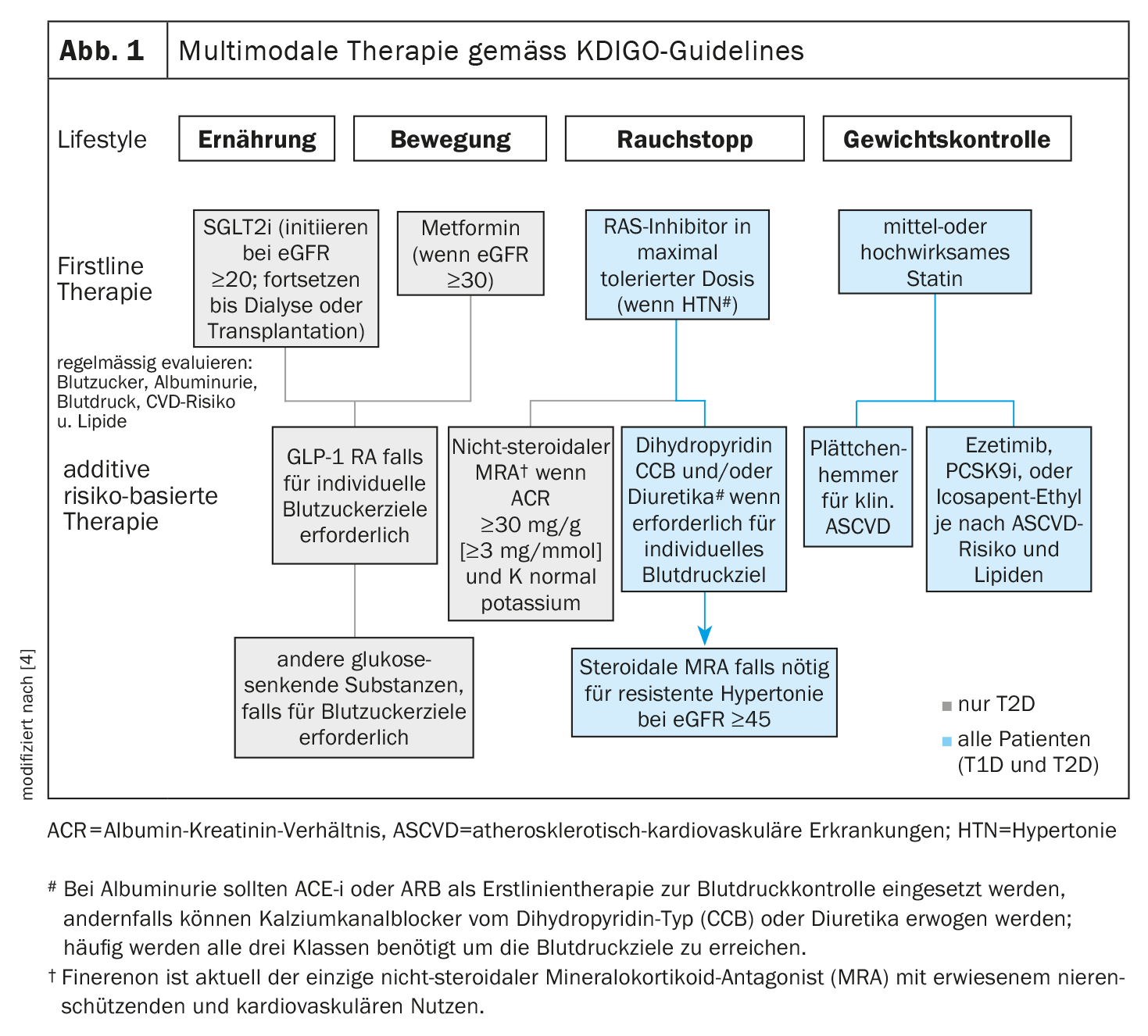
The fact that a revised version of the KDIGO guidelines issued in 2020 is already available in 2022 reflects the rapid progress in the field of treatment of diabetes-associated nephropathy [4]. The KDIGO propagates a multimodal therapy approach (Fig. 1).
- All patients should pay attention to lifestyle factors such as exercise, diet, and smoking cessation. First-line drug therapies must be selected according to individual clinical characteristics.
- Glycemic control in type 2 diabetes (T2D) is based on a combination of metformin and sodium-glucose cotransporter-2 inhibitors (SGLT-2-i). For patients in whom SGLT-2-i and metformin are not effective in achieving glycemic goals, glucagon-like peptide-1 receptor agonists (GLP-1 RA) are the preferred glucose-lowering medication.
- In patients with albuminuria and hypertension (HTN), inhibition of the renin-angiotensin system (RAS) is advised. A statin is recommended for all patients with T1D or T2D and CKD.
- A nonsteroidal mineralocorticoid receptor antagonist (ns-MRA) may be added to first-line therapy in patients with T2D and a high residual risk of CKD progression and cardiovascular events**.
- Aspirin is generally used for secondary prevention in patients with established cardiovascular disease throughout life, unless contraindications exist, and may be considered for primary prevention in patients at high risk for atherosclerotic cardiovascular disease (ASCVD).
** Indicator of high residual risk for progression of CKD and cardiovascular events: persistent albuminuria (>30 mg/g [>3 mg/mmol]).

SGLT-2-i: the eGFR threshold was lowered
Patients with T2D, CKD, and an eGFR ≥20 ml/min per 1.73 m² have now been extensively studied in RCTs on SGLT-2-i. Based on these studies, the strong recommendation is reiterated to treat patients with T2D and CKD with an SGLT-2-i, regardless of albuminuria. An important change is the lower eGFR threshold at which SGLT-2-i should be initiated. The 2022 guideline recommends implementation of an SGLT-2-i for patients with T2D and CKD who have an eGFR ≥20 ml/min per 1.73 m², as opposed to ≥30 ml/min per 1.73 m² in the 2020 guideline [4].

The studies Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease. (DAPA-CKD) and Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) enrolled CKD patients with an eGFR of up to 25 ml/min per 1.73 m² [5,6].
The Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY), conducted exclusively with CKD populations and terminated early because of benefit, also included participants with an eGFR ≥20 ml/min per 1.73 m² [7]. Subgroup analyses of individual studies and meta-analyses have shown renal and cardiovascular benefits in all eGFR categories, including participants with eGFR <30 ml/min per 1.73 m² and those without albuminuria [8,9].

GLP-1 receptor agonists: new insights.
In 2021, the results of a new large endpoint study on efpeglenatide were published and have been incorporated into the new KDIGO guidelines. The AMPLITUDE-O (Effect of Efpeglenatide on Cardiovascular Outcomes) study supported the evidence for the cardiovascular benefits of GLP-1 RA and reinforced the hypothesis that GLP-1 RA may also improve renal outcomes [2]. Accordingly, GLP-1 RA remain the recommended second-line therapy for glycemic control in T2D and CKD. The proven cardiovascular benefit of GLP-1 RA was demonstrated for all categories of eGFR and served as the main rationale for recommending GLP-1 RA as the preferred drug to lower blood glucose in patients with T2D and CKD who did not achieve their glycemic goals despite the use of SGLT-2-i and metformin (or in whom SGLT-2-i and/or metformin could not be used).
Finerenone – non-steroidal mineralocorticoid antagonist as latest player
Also effective in preventing the progression of diabetic kidney disease is the novel nonsteroidal selective mineralocorticoid receptor antagonist finerenone [1]. This was demonstrated by the two large clinical trials FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) [10] and FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) [11] as well as the combined analysis FIDELITY based on them [12]. The most important results at a glance:
In the FIDELIO-DKD trial, finerenone significantly reduced the incidence of both the primary composite renal outcome (renal failure, sustained decline in eGFR by ≥40%, or death due to renal disease; hazard ratio [HR]: 0.82; 95% CI: 0.73-0.93) and the secondary composite cardiovascular outcome (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure; HR: 0.86; 95% CI: 0.75-0.99). [10].
In FIGARO-DKD, finerenone significantly reduced the combined cardiovascular endpoint (HR: 0.87; 95% CI: 0.76-0.98) [11].
In the FIDELITY trial, overall cardiovascular outcomes were reduced in patients treated with finerenone (HR: 0.86; 95% CI: 0.78-0.95), with no significant heterogeneity in baseline patient characteristics [12].
Patients treated with finerenone were also less likely to experience renal failure, a decrease in eGFR of more than 57%, or renal death (HR: 0.77; 95% CI: 0.67-0.88), and less likely to experience renal failure defined as initiation of chronic dialysis or renal transplantation (HR: 0.80; 95% CI: 0.64-0.99).
Literature:
- “World Diabetes Day: dialysis is not an inevitable fate in people with diabetes mellitus!”, German Society of Nephrology, Nov. 11, 2022.
- Gerstein HC, et al: N Engl J Med 2021; 385: 896-907.
- Position Paper Cooperation Diabetology/Nephrology. German Diabetes Society/German Society of Nephrology/Association of Senior Hospital Nephrologists 2018. www.ddg.info,(last accessed Jan. 10, 2023).
- Rossing P, et al: Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney Int 2022; 102(5): 990-999.
- Bhatt DL, et al: N Engl J Med 2021; 384: 117-128.
- Heerspink HJL, et al: N Engl J Med 2020; 383: 1436-1446.
- EMPA-Kidney Collaborative Group: Nephrol Dial Transplant 2022; 37; 1317-1329.
- Bakris G, et al: Clin J Am Soc Nephrol 2020; 15: 1705-1714.
- Chertow GM, et al: J Am Soc Nephrol 2021; 32: 2352-2361.
- Bakris GL, et al: N Engl J Med 2020; 383: 2219-2229.
- Pitt B, et al: N Engl J Med 2021; 385: 2252-2263.
- Rossing P, et al: FIDELIO-DKD and FIGARO-DKD Investigators: Diabetes Care. Published online, August 15 (2022), 10.2337/dc22-0294.
- Seidu S, et al: 2022 update to the position statement by Primary Care Diabetes Europe: a disease state approach to the pharmacological management of type 2 diabetes in primary care. Prim Care Diabetes 2022; 16(2): 223-244.
- “Chronic Kidney Disease-associated Pruritus: From Epidemiology To Treatment,” 11/25/2021, www.emjreviews.com, (last accessed 12/14/2022).
- Sukul N, et al: Kidney Med 2020; 3(1): 42-53.e1.
- Yan B, et al: J Am Heart Assoc 2021; 10(7): e016201.
- “CHMP Meeting Highlights February 2022,” 3/17/2022, www.basg.gv.at, (last accessed 12/14/2022).
- Fishbane S; KALM-1 Trial Investigators: N Engl J Med 2020; 382(3): 222-232.
- American Society of Nephrology: Abstract FR-OR24, www.asn-online.org (last accessed Dec. 14, 2022).
HAUSARZT PRAXIS 2023; 18(1): 20-21











