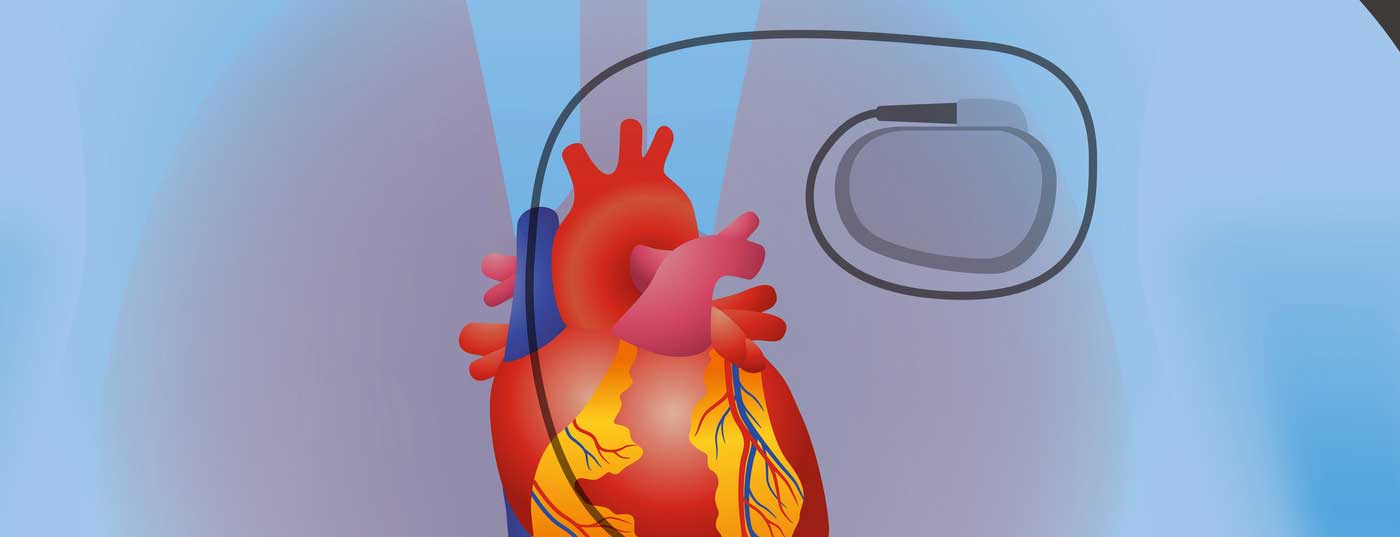
It can be dangerous when a patient with cardiac device needs an MRI. Among other things, the electrodes may heat up, an electrical reset is conceivable and the battery status threatens to change.
In an MRI, energy is delivered to the body in the form of a strong static magnetic field as well as electromagnetic pulses (radio frequency pulses). Then, in very simplified terms, the signals sent back from the tissue are measured, resulting in an image. Depending on the tissue type, the magnetization distributions differ, depending on structure, function and metabolism. Compared to CT, for example, the soft tissue contrast is incomparably higher. The magnetic field strengths achieved in MRI (static magnetic field) are 1.5-3 Tesla. As a comparison, the earth’s magnetic field has a strength of about 50 microtesla, a household magnet is in the millitesla range.
So you can imagine the force on implantable devices once you’re in the vicinity of an MRI. The main problem is not even the static magnetic field, which may lead to device displacement or electrode dislocation, or the gradient magnetic field, which may induce ventricular or atrial capture with corresponding arrhythmias by current induction in the electrode, but mainly the radiofrequency pulse. This can, for example, heat up the electrodes, with corresponding thermal damage, or it causes an electrical reset due to high electromagnetic interference. The battery status may also change or there may be “oversensing” with inhibition of pacing as well as inadequate shock delivery by an ICD.
Thermal damage from an MRI or radiofrequency is primarily due to temperature increases at the tip of the electrode. In studies, 0.5 Tesla devices already heated up to approx. 24°C [1], and 1.5 Tesla even up to 63°C [2]. With electrodes connected to the pacemaker of about 40-60 cm in length, i.e. in the normal case, the temperature increase is not dramatic, but with free electrodes in the tissue, the temperature increases significantly, especially at the tip [3].
Now, more and more MRI systems are being installed across Europe, including those with ever increasing Tesla power. At the same time, the number of pacemaker and ICD first implantations is increasing, so the question of an MRI examination in a patient with CIED is becoming more frequent. An estimated 50-75% of these patients will have an MRI indication during the lifetime of their device. What to do?
Systems suitable for MRI
As a possible solution to the problem, pacemakers and later also ICDs that are MRI-compatible have existed for a few years. Strictly speaking, these are referred to as “MR-conditional systems” because they can only exist in an MR environment under certain conditions, e.g., only with 1.5 Tesla devices or excluding a thoracic MRI (cranial and lumbar spine MRI). A list of MR-capable devices with the corresponding conditions or limitations can be found at www.pacemaker.ch. Of course, the battery and electrode must be MR-compatible.
And what about the others? The MagnaSafe register
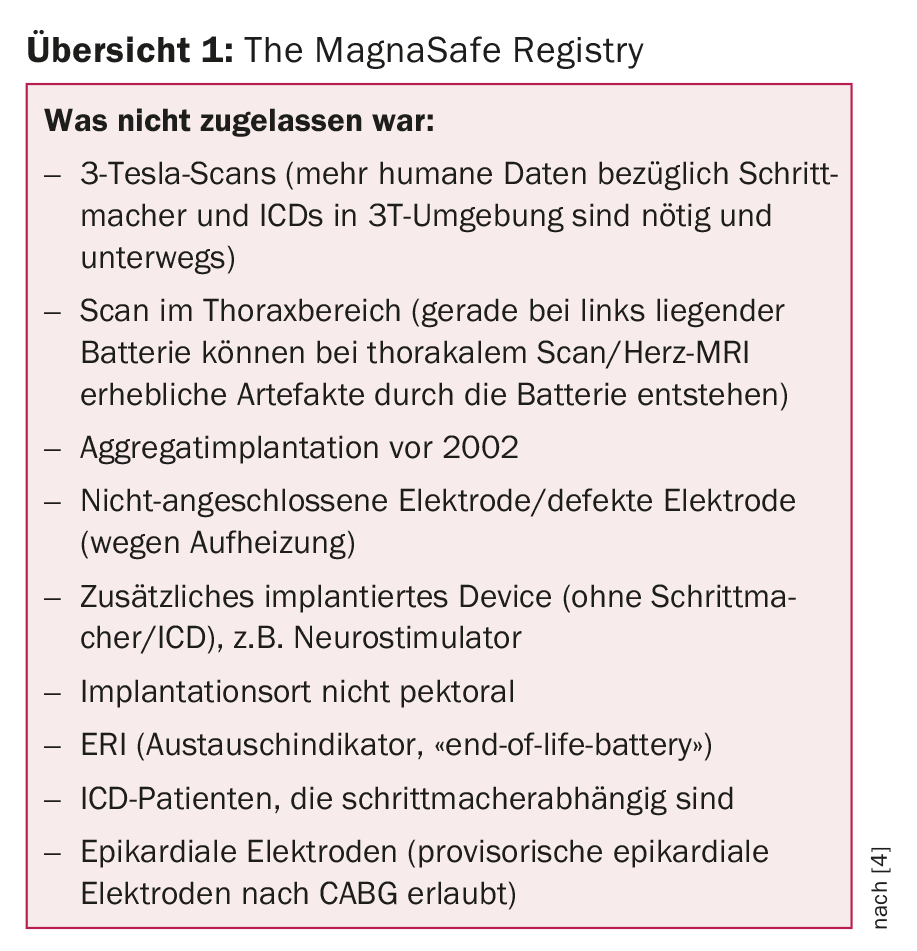
Data from the MagnaSafe registry – published this year [4] – show: Under clearly defined conditions, an MRI examination also works with the remaining devices not specifically designed for MR suitability. In total, the study performed 1000 MRI scans (1.5 Tesla) in pacemaker patients and 500 in ICD patients. The aggregate implantation had to have taken place after 2001. The exclusion criteria are shown in the overview 1. If there is a corresponding indication, one should follow it.
The basic finding of the study: MRI imaging in patients with “non-conditional” systems appears to be quite safe, provided that inclusion and exclusion criteria are followed, as well as a structured workflow for device control/programming before and after MRI. In detail:
- No deaths, ventricular arrhythmias, electrode failure, or capture failure during MRI examination.
- Six patients with atrial fibrillation/flutter during or immediately after MRI (five of whom had a relevant history).
- Six partial electrical resets (patient information/electrode information erased on device, partially in reset mode, etc.).
- An ICD could no longer be interrogated and therefore had to be replaced on an emergency basis. However, in this case, antitachycardia therapy was forgotten to be issued before the scan (protocol violation). During the scan, the device falsely detected ventricular fibrillation, failing to charge the capacitor in the MRI. The device needed a system reset by the company (but was then of course no longer in the patient).
Consensus Statement
The US Heart Rhythm Society issued a consensus statement on the topic a little later in the year [5]. Therein, MRI is considered reasonable for “non-conditional” devices, provided it is clearly the best investigation in this situation, there are no broken, epicardial, or free leads, and most importantly, following an institutionalized protocol with a responsible MR and device specialist.
According to Dr. Zbinden, the standardized procedure (whether MRI-ready or not) includes coordination between the device consultation and radiology to interrogate and program the device before and after MRI, i.e., reprogramming to asynchronous mode (or “off” if the patient’s own rhythm is sufficient) and, in the case of ICDs, turning off tachy detection before the examination and reprogramming afterwards. Certain devices will offer this automatically in the future: One programs the MRI detection mode, for example, 48 hours to 14 days before examination and the device then automatically switches to asynchronous mode during the scan and then back.
In any case of non-suitable equipment, a critical discussion of the MRI indication with the radiologist and signing of a consent form by the patient are indicated.
By the way: If there is a clear MR indication, the examination can also be performed directly after implantation of a “non-conditional” system (class IIa), according to the American guideline [5].
Source: Cardiology Update Refresher, November 17-18, 2017, Zurich.
Literature:
- Sommer T, et al: MR imaging and cardiac pacemakers: in vitro evaluation and in vivo studies in 51 patients at 0.5 T. Radiology 2000 Jun; 215(3): 869-879.
- Achenbach S, et al: Effects of magnetic resonance imaging on cardiac pacemakers and electrodes. Am Heart J 1997 Sep; 134(3): 467-473.
- Langman DA, et al: Pacemaker lead tip heating in abandoned and pacemaker-attached leads at 1.5 Tesla MRI. J Magn Reson Imaging 2011 Feb; 33(2): 426-431.
- Russo RJ, et al: Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. N Engl J Med 2017 Feb 23; 376(8): 755-764.
- Indik JH, et al: 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm 2017; 14(7): e97-e153.
CARDIOVASC 2017; 16(6): 31-32