The scientific advances of the last few decades have contributed significantly to our understanding of the pathogenesis of acne and the further development of treatment options based on this. According to current understanding, acne vulgaris is the result of a complex interaction between genetic factors and environmental influences. This is supported by current study findings on comedogenesis and bacterial colonization of acne skin.
Acne vulgaris is known to be a keratinization disorder of the sebaceous follicles with increased sebum production that occurs predominantly during puberty and is associated with androgenic sebaceous gland hyperfunction. The clinical picture is characterized by comedones and secondary inflammatory efflorescences such as papules, pustules and abscessed nodules, which are predominantly localized on the face and upper trunk [1–3]. “The pathogenesis of acne is relatively complex and multifaceted,” summarized Prof. Dr. Vincenzo Bettoli, MD, Università di Ferrara (I) [4]. The following factors play an important role: genetic predisposition, hormonal androgens, sebaceous gland hyperplasia and increased sebum production, hyperkeratosis, microbial hypercolonization, inflammation and immune response [1,6]. While the colonization of the hair sebaceous gland unit with Propionibacterium was previously regarded as the main cause of acne, the contribution of host-specific factors that enable bacterial growth and the immune response against bacterial components has recently come into focus [7]. Many of these traits have a genetic basis involved in either the regulation of immune responses or steroid hormone metabolism [7].
Genetic factors predispose to the occurrence of acne
“The sebaceous glands play a very important role,” emphasized Prof. Bettoli [4]. It is not only about the amount of sebum production, but also about the type of sebum – for example, a number of fatty acids are produced that have a comedogenic effect. “Sebaceous glands can also induce inflammatory processes,” explained the speaker [4]. Genetic factors in the etiology of acne appear to have an indirect influence on sebum production, distribution and severity of lesions [6,8]. Studies confirm that identical twins suffering from acne have a similar sebum production rate. It is also known that acne patients with parents who have increased sebum production and severe forms of the disease can also be expected to have this [8,9]. In recent years, researchers have identified genes that are associated with acne vulgaris and can influence the progression and prognosis of the disease. An overview article published in 2023 shows what these are, including genes coding for interleukins (IL), tumor necrosis factor (TNF), RETN, matrix metalloproteinases (MMPs) and TIMPs [10].
Bacterial colonization: What is the current state of knowledge?
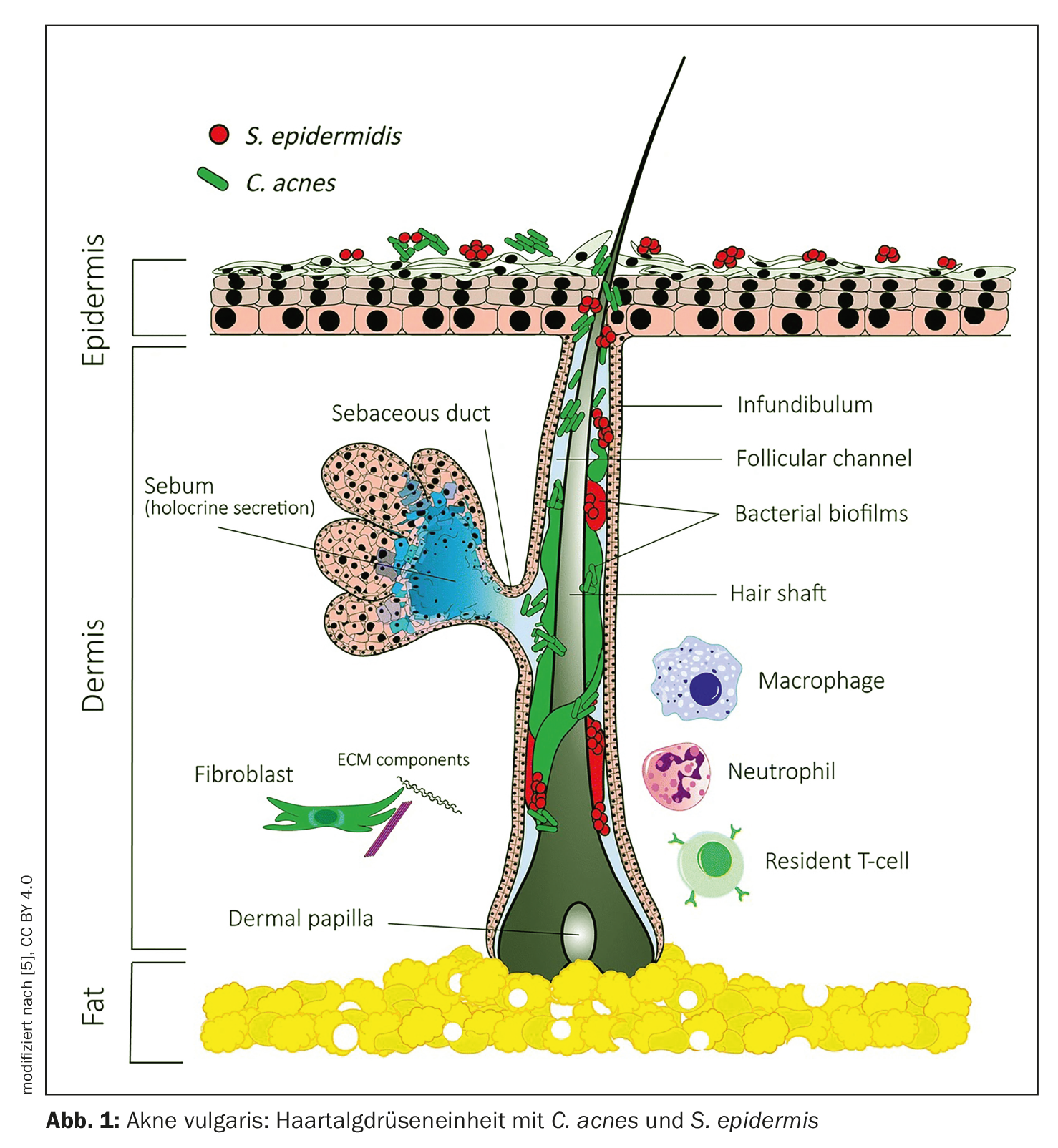
The commensal bacterium Propionibacterium acnes (P. acnes) is now known as Cutibacterium acnes (C. acnes) and can occur as an opportunistic pathogen in acne vulgaris [12]. Contrary to previous assumptions, the proliferation of C. acnes is not the cause of acne, as patients with acne do not have more C. acnes in their follicles than people without acne [12]. Rather, the loss of microbial diversity in the skin together with the activation of innate immunity could lead to this chronic inflammation. This is the conclusion of a review by Dréno et al. out. Besides C. acnes appears to have other players that play a role in the pathophysiology of acne, for example Staphylococcus epidermidis (S. epidermidis) (Fig. 1) . Recent research suggests that acne may be the result of an unbalanced equilibrium between C. acnes and S. epidermidis based on specific interactions [13]. Thus, the role of S. epidermidis in acne is to limit C. acnes overgrowth and inflammation.
Nutrition: Evidence does not allow for a generally valid recommendation
It is known that androgens and stress are important factors, and there is also a theory that diet has an influence on the development of acne. However, according to Prof. Bettoli, no general evidence-based recommendation for a low-glycaemic diet can be derived from the current data situation [4]. According to a systematic review published in JAAD in 2022, although there is evidence that a high glycemic index and increased glycemic load have a moderate acnegenic effect and that increased milk consumption can have an acnegenic effect in certain patient populations, the influence of diet on acnegenesis is subject to factors such as gender and other patient characteristics [11]. The conclusion of this review article is that further randomized studies are needed to fully characterize possible associations.
How microcomedones and inflammatory efflorescences develop
In connection with a proliferation and retention hyperkeratosis, as well as increased sebum production and altered lipid composition of the sebum, invisible bulges of the hair follicles, the microcomedones, occur [6,14,15]. The development of secondary, inflammatory efflorescences is associated with microbial hypercolonization of C. acnes. C. acnes is a gram-positive rod bacterium [14,16]. If there is inflammation within the follicle, small, raised, inflammatory, erythematous papules develop. If the inflammatory follicle wall ruptures, lipids and corneocytes are released, which further aggravate the inflammation, resulting in red pustules filled with pus [9,14,15]. As the pustules progress, inflammatory nodules (>5 and <10 mm in diameter) or lumps (>10 mm in diameter) can form. Severe forms of acne also manifest as deep, melting abscesses with fistula tracts that can flare up repeatedly [9,15]. Scars and keloids form in the case of progression with nodule formation or mechanical impact, whereby these are based on an imbalance of MMPs and TIMPs [4].
| Informative in vivo study on comedogenesis Ten 12-30-year-old patients with mild to moderate acne who were not being treated with topical or systemic therapies were recruited for a study published in JEADV in 2019. The aim was to characterize the morphological changes with the help of confocal laser scanning microscopy (Reflectant Confocal Microscopy, RCM) and dynamic optical coherence tomography (D-OCT). For this purpose, a 4× 4 mm area of skin on the face was selected that had no acne lesions at the start of the study. Over a period of 6 weeks, a series of standardized clinical images as well as RCM and D-OCT images were acquired and evaluated on a weekly basis. The analyses showed that the appearance of an acne lesion is preceded by a proliferation of large, bright follicles in the area corresponding to infundibular keratinization, followed by an increase in inflammatory parameters, such as a proliferation of small bright cells (RCM) and a vascular network (D-OCT), which return to normal after the acute inflammation subsides. In summary, the dynamics of acne skin are complex and appear to be characterized by the early increase of dysmorphic hair sebaceous gland units and hyperkeratinization of the acroinfundibulum of the pilosebaceous duct before inflammatory events occur around the follicle. Hyperkeratinization and inflammatory processes can lead to a vicious circle that perpetuates acne. |
| to [20] |
What are the most important treatment options today?
Acne is a very individual disease, but there are some evidence-based treatment options. The primary aim of acne therapy is to alleviate the symptoms and prevent acne scars. For all acne patients, the use of Lubex® (1×/d) is recommended for basic care or benzoyl peroxide (Lubexyl®) for thick skin and severe infestation [21,22]. Various treatment options are available for the actual local treatment of mild to moderate acne [17]. These include clindamycin (Dalacin® T emulsion) and, for moderate acne, azelaic acid as an add-on (e.g. Skinoren® cream, 2×/d) and topical retinoids (e.g. Epiduo®, Epiduo forte® or Aklief®) [21,22]. The use of adapalene (Differin® Gel, 1×/d) has proven effective for acne comedonica [17,21,22]. Acne tarda can be treated in the same way as juvenile acne. Systemic therapy is indicated for severe forms of acne or indications of a scarring tendency [22]. For moderate to severe inflammatory acne, systemic antibiotics (lymecycline 1× 300 mg/d, doxycycline 2× 50 mg/d, tetracycline 1× 100 mg/d) are recommended for a period of 1-3 months, whereby these should be combined with topical therapy. If there is no pre-existing liver disease, no laboratory checks are necessary during this short treatment period, but care should be taken to ensure adequate UVA protection. The use of systemic retinoids (isotretinoin) may be considered for severe papulopustular/nodular acne that does not respond adequately to topical and systemic antibiotic therapy. Isotretinoin (Roaccutane®) is often the only effective medication for acne tarda. The initial dose is 10 mg/d with an increase in the further course up to a maximum of 0.5-1 mg/kg bw over at least six months (cumulative target dose: 120-150 mg/kg bw).
Literature:
- Sterry W, Burgdorf W, Paus R: Acne. In: Checklist Dermatology. 6th ed. Georg Thieme Verlag. Stuttgart; 2010; pp. 539-544.
- Klausner M, Hausar G: Acne vulgaris. In: Special pathology: for medical masseurs and medical staff. Facultas Verlag. Vienna; 2009, pp. 56-57.
- Altmeyer P, Hoffmann K: Acne. In: Basiswissen Dermatologie: Eine vorlesungsorientierte Darstellung. W3L Publisher. Herdecke, Bochum; 2006, pp. 347- 351.
- “Pathophysiology of acne and rosacea”, Prof. Dr. Vincenzo Bettoli, MD, Session ID D1T11.1, Acne and rosacea, EADV Annual Meeting 11-13.10.2023
- O’Neill AM, Gallo RL: Microbiome 2018; 6: 177, https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-018-0558-5,(last accessed 28.11.2023)
- Stauber G, Patscheider R: Swiss Journal of Nutritional Medicine; 03/2008.
- Elsaie ML, Aly DG: Adv Exp Med Biol 2022; 1367: 137-154.
- Borelli C, et al.: In: Plewig G, Thomas P (eds.): Fortschritte der praktischen Dermatologie und Venerologie 2006. Springer Medizin Verlag. Heidelberg; 2007, pp. 485-492.
- Kerscher M, Williams S, Trüeb RM: Seborrheic acne-prone skin. In: Dermatocosmetics. 2nd ed. Steinkopff Verlag; 2009, pp. 71-82.
- Zhang H, Zhang Z: Int J Gen Med 2023 Aug 28; 16: 3843-3856.
- Meixiong J, et al: Diet and acne: A systematic review. JAAD Int 2022; 7: 95-112.
- Dréno B, et al: JEADV 2018; 32 Suppl 2: 5-14.
- Claudel JP, et al: Dermatology 2019; 235(4): 287-294.
- Degitz KK, Krauß HJ: Pathogenesis, clinic and pharmacotherapy of acne. GoviVerlag. Eschborn; 2004.
- 15 Girbig P., Siemann-Harms U.: Acne vulgaris. In: Moll I. (ed.): Duale Reihe – Dermatologie. 6th edition. Georg Thieme Publishers. Stuttgart; 2005, pp. 472-479.
- Goerdt S: Acne. In: Wehling M. (ed.): Clinical Pharmacology. 2nd ed. Georg Thieme Verlag. Stuttgart; 2011, pp. 628 – 635.
- Nast A, et al: JEADV 2016; 30(8): 1261-1268.
- Zouboulis CC: Modern acne therapy. Act Dermatol 2003; 29: 49-57.
- Strom K, Abeck D: Acne. In: Abeck D., Cremer H. (eds.): Common skin diseases in childhood. 3rd ed. Steinkopff Verlag. Darmstadt; 2006, pp. 1-9.
- Manfredini M, et al: JEADV 2019; 33(9): 1768-1774.
- Swissmedic: Arzneimittelinformation,
www.swissmedicinfo.ch,(last accessed 29.11.2023). - Factsheet Acne, last update: 11/2021, www.medix.ch,(last accessed 29.11.2023).
DERMATOLOGIE PRAXIS 2023; 33(6): 36-37 (published on 12.12.23, ahead of print)