Which drugs are suitable for neoadjuvant radiochemotherapy? If there is a complete clinical response, should we wait or operate? And which patients benefit from adjuvant treatment? These were some of the questions discussed at the EORTC GICC in St. Gallen at the beginning of March.
(rs) “In up to 30% of those with advanced distal rectal cancer, preoperative (neoadjuvant) radiochemotherapy (RCT) can achieve a complete pathologic (pCR) and clinical response (cCR), potentially avoiding radical surgery,” said Prof. Angelita Habr-Gama, MD, Sao Paulo, at the EORTC GICC in St. Gallen. Aiming to standardize the approach to tumor diagnosis, Prof. Habr-Gama and colleagues defined clinical and endoscopic criteria for clinical response and, based on these, further procedures [1]. In addition to the standardized procedure, the timing of tumor assessment also plays an important role. “Several studies show that the assessment should be done at the earliest seven to eight weeks after the end of neoadjuvant RCT,” Prof. Habr-Gama said. She referred to a study by Kalady et al that showed that a time interval of more than eight weeks between the end of neoadjuvant RCT and surgery was associated with significantly higher pCR [2]. Overall (OS) or disease-free survival (DFS) was not affected by delayed tumor assessment in studies.
“Watch and Wait” or operate immediately?
The prerequisite for the “Watch and Wait” protocol presented by Prof. Habr-Gama and used since 1991 is a complete clinical response after neoadjuvant RCT (Fig. 1) .
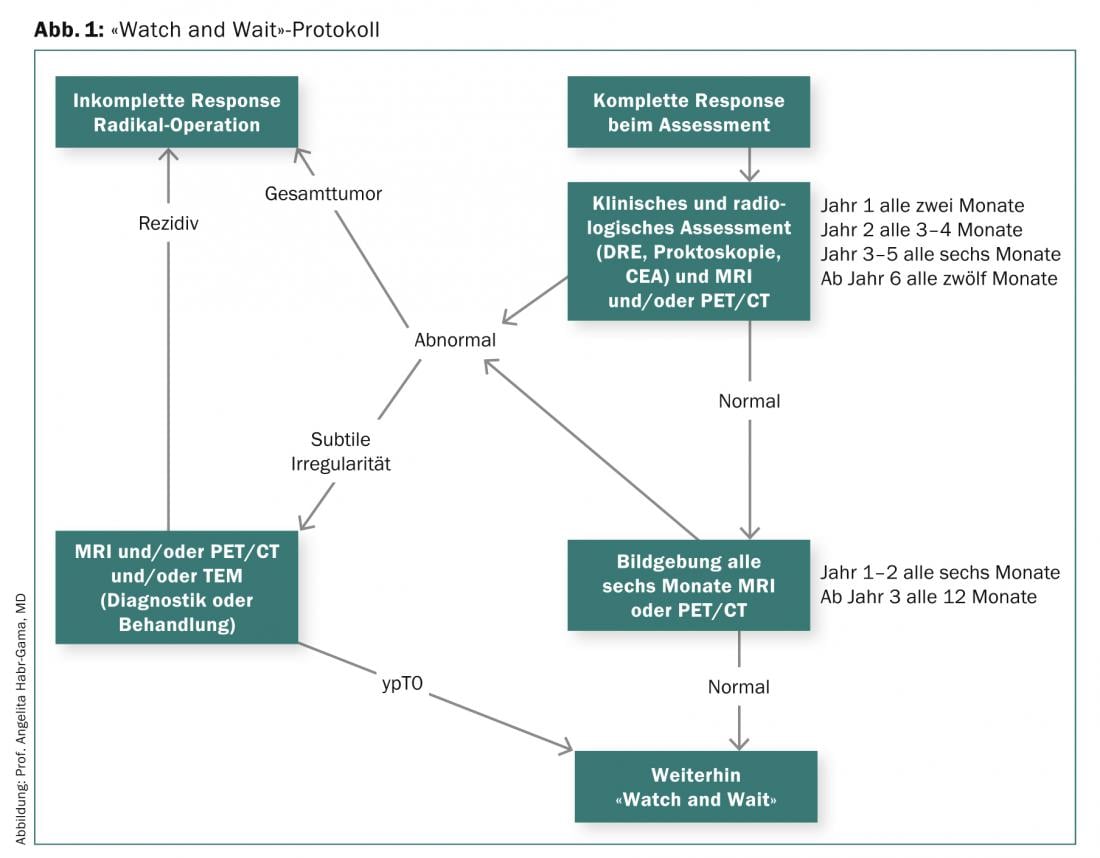
As shown by the long-term results of a study of 265 patients with distal rectal cancer who underwent either radical surgery (incomplete response) or follow-up (cCR) after neoadjuvant RCT, the overall and disease-free survival rate was 84 vs. 97.7%. After five years, overall survival was 88 in the resection group and 100 in the observation group, and disease-free survival was 83 and 92%, respectively. To increase the number of patients in whom radical surgery can be avoided, Prof. Habr-Gama and colleagues investigated the impact of prolonged neoadjuvant RCT with higher radiation dose, more frequent chemotherapy cycles, and a longer interval to tumor assessment [3]. The investigated therapy regimen initially led to cCR in 68% of patients. Within the first twelve months, 17 and during the course (>12 months) another 10% developed a local recurrence. Half of the included 70 patients did not require surgery in the long-term (median follow-up 56 months).
Optimal partners for neoadjuvant RCT
The answer to the question of which chemotherapy is best suited for combination with radiotherapy in the adjuvant treatment of locally advanced rectal cancer is complicated by the different administration of fluoropyramidines. “We know from postoperative studies that 5-FU plus radiotherapy is an active treatment and that continuous i.v. administration during radiation is superior to bolus injections,” said Prof. Hans-Joachim Schmoll, MD, of Germany’s University of Halle. Prof. Schmoll answered whether the oral 5-FU prodrug capecitabine is preferable to the i.v. form of 5-FU in perioperative treatment based on a publication by Hofheinz and colleagues. This showed a significant survival benefit for capecitabine at five years (76 vs. 67% p=0.0004) [4]. The results of the NSABP-R-04 trial published at ASCO 2013, which investigated treatment with capecitabine plus radiotherapy (RT) in two of the total four arms and an RCT with continuous low-dose infusion 5-FU, showed a comparable outcome [5]. “The study results show a comparably effective treatment,” Prof. Schmoll said. “However, because of its easier administration, capecitabine should be preferred to 5-FU.”
The question of fluoropyramidine-based RCT plus oxaliplatin for neoadjuvant RCT in locally advanced colon cancer was evaluated using five trials: STAR, ACCORD/Prodige2, NSABP R-04, ARO/CAO/AIO 04, PETACC-6. Only the ARO/CAO/AIO-04 trial showed a significantly higher pCR (17 vs. 13%, p=0.038) [6]. All other studies were negative in this regard. However, as shown by the aforementioned NSABP-R-04 trial, which examined a neoadjuvant RCT with fluoropyrimidines plus oxaliplatin in the remaining two arms, toxicity increased significantly with the combination.
A neoadjuvant RCT with capecitabine and irinotecan has been investigated in smaller trials. These showed pCR rates between 9 and 27% and a high R0 resection rate. The combination with the VEGF inhibitor bevacizumab initially appeared promising. “The results from phase I and II studies, with pCR rates ranging from 16% to 36%, do not suggest that the combination with bevacizumab contributes significantly to improved outcome,” Prof. Schmoll said. The same applies to the EGFR inhibitor cetuximab.
Adjuvant chemotherapy
Far less clear-cut was the question of postoperative (adjuvant) chemotherapy in the treatment of rectal cancer. While there is widespread consensus that adjuvant chemotherapy is beneficial in high-risk patients. However, this indirect conclusion is drawn from older studies with patients who had largely not received neoadjuvant therapy. In contrast, it is known from the EORTC trials that patients who had responded well to neoadjuvant therapy benefit most from adjuvant CT [7]. Six months of therapy with 5-FU or capecitabine is recommended for adjuvant treatment. Information on the combination with oxaliplatin in adjuvant CT will be provided by initial results from the PETACC-6 and ARO/CAO/AIO-04 trials. These are expected to be presented at ASCO 2014.
Source: “Issues in combined modality treatment for rectal cancer”,2nd St. Gallen EORTC (European Organisation for Research and Treatment of Cancer) Gastrointestinal Cancer Conference (GICC) 2014, March 7, 2014, St. Gallen.
Literature:
- Habr-Gama A, et al: Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum 2010; 53(12): 1692-1698.
- Kalady MF, et al: Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 2009; 250(4): 582-589.
- Habr-Gama, et al: Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum 2013; 56(10): 1109-1117.
- Hofheinz RD, et al: Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012; 13(6): 579-588.
- Roh MS, et al: The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J Clin Oncol 29: 2011 (suppl; abstr 3503).
- Rödel C, et al: Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012; 13(7): 679-687.
- Collette L, et al: Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007; 25(28): 4379-4386.
InFo ONCOLOGY & HEMATOLOGY 2014; 2(4): 25-27.











