The determination of psychotropic drugs in the blood (therapeutic drug monitoring, TDM) contributes to an optimal and personalized therapy of the psychiatric patient. This article presents a brief introduction to the renewed consensus guidelines of the AGNP-TDM group.
Blood level determination of psychotropic pharmaceuticals serves to elucidate pharmacokinetic characteristics in individual patients and thus contributes to therapy optimization. Guidelines for Therapeutic Drug Monitoring (TDM) published over the past several decades have come virtually exclusively from the TDM Group of the Association for Neuropsychopharmacology and Pharmacopsychiatry (AGNP). Following the TDM Group guidelines published in 2004 [1] and 2011 [2], an updated English-language version, expanded to include a large number of drugs, was presented in 2017 [3] and is now available in both comprehensive [4] and abbreviated versions [5] in German. The abridged version of the original guideline presented here (in electronic form in both German and French) is intended to encourage readers to return to the original English version: it is freely available on the website www.agnp.de and offers the potential to improve the efficacy and tolerability of neuropsychopharmacotherapy using TDM for the direct benefit of patients and to reduce the cost of therapy. To illustrate the practical application of this consensus guideline, a previously unpublished clinical situation is presented for illustration (Patient A., box).
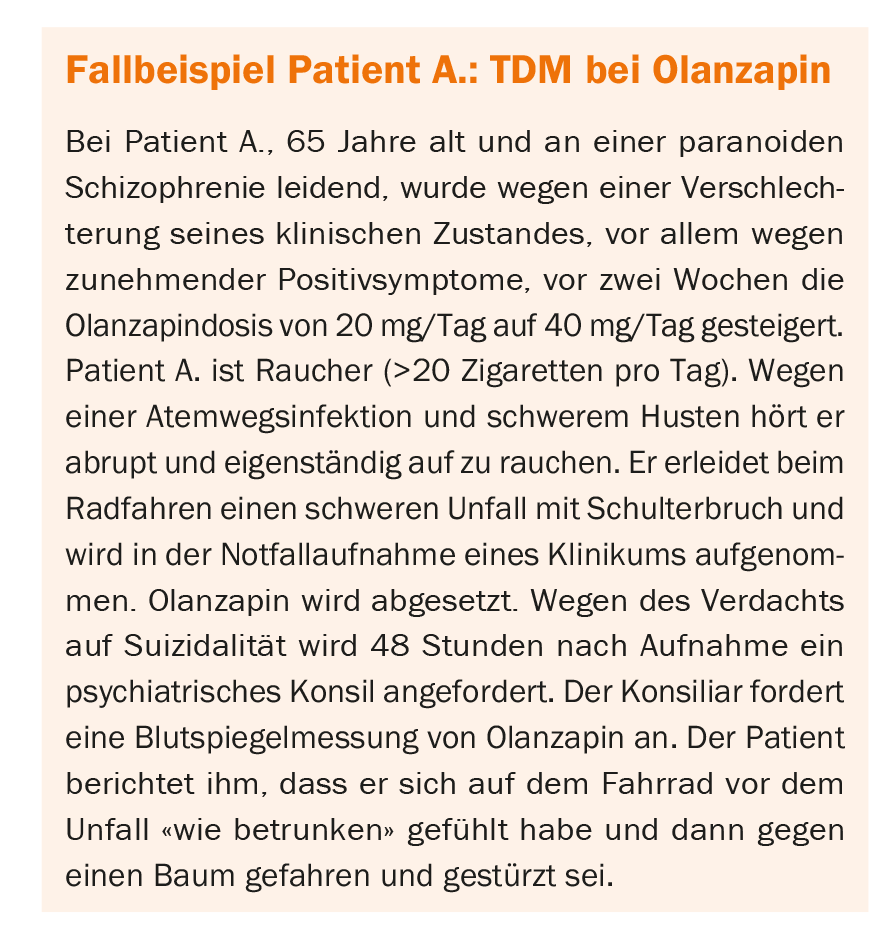
Indications for the TDM
Measurement of drug blood levels should not be performed without precise indication. Thus, Table 1 presents a selection of situations in which TDM proves useful.
In patient A, blood level measurement was indicated for several reasons: adverse drug reaction at recommended dose, acute infection and treatment with substrate of CYP1A2 (here olanzapine), and suspected drug interaction (here smoking cessation). A blood level measurement would have been indicated before the dose increase from 20 mg to 40 mg due to the return of symptomatology under adequate dose.
Recommendation grades
There is agreement on the need for lithium determinations because lithium has a narrow therapeutic range in that too low blood levels are associated with therapeutic inefficacy, whereas too high blood levels are associated with a risk of toxicity. For other drugs, which, for example, like agomelatine, have an exceedingly short elimination half-life, TDM is generally of little use. The AGNP-TDM group has therefore defined four levels of recommendation:
- Grade 1: strongly recommended (e.g. lithium)
- Grade 2: recommended (e.g. risperidone)
- Grade 3: useful (e.g. fluoxetine)
- Grade 4: potentially useful (e.g. agomelatine)
TDM is strongly recommended for 19 of the 154 drugs studied.
Back to our case study, patient A.: In the case of olanzapine, TDM with recommendation grade 1 is strongly recommended (Tab. 2), although in individual cases it may also be useful to include not only the parent substance but also the non-active metabolite N-desmethylolanzapine.
However, not every laboratory offers this determination, as problems may arise in obtaining reference substances.
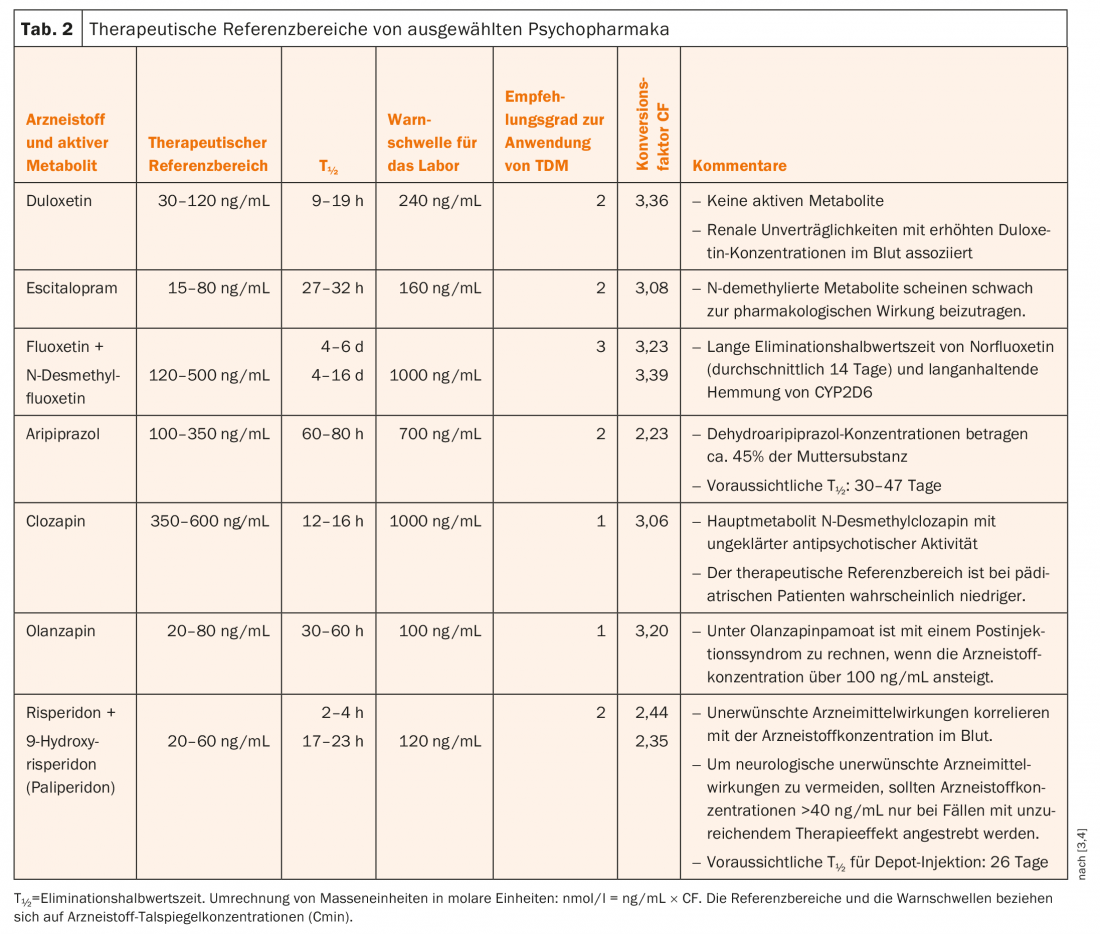
Therapeutic and dose-related reference ranges
Two initial questions arise for the interpretation of TDM results:
- Is the measured drug concentration within or outside the therapeutic reference range?
- Does it correspond to that expected in patients without comedication and without genetic peculiarities at the prescribed dosage?
Table 2 shows the therapeutic reference ranges of blood concentrations of selected drugs. These are concentrations at which treatment response and good tolerability can be expected.
In patient A, the following blood level was measured under the olanzapine dose of 40 mg: Olanzapine 118 ng/mL (therapeutic reference range 20-60 ng/mL). The value was above the therapeutic reference range, even above the laboratory’s warning threshold (100 ng/mL), i.e. in the potentially toxic range. Therefore, the findings from the laboratory were communicated to the treating physicians by telephone immediately after receipt of the value. In addition, the laboratory calculated and reported that the blood level at the time of the accident must have been above 200 ng/mL.
Table 3 presents pharmacokinetic data of a selection of antidepressants and antipsychotics. From the dose and the so-called DRC (“dose related concentration”) factors, it is possible to calculate which blood level range can normally be expected for a given dose. DRC factors were calculated from pharmacokinetic data (clearance, bioavailability, and elimination half-life) taken from the current literature. For DRC, the mean (“medium”) value is given in Table 3, as well as the “low” and “high” limits, which correspond to the range of the mean ± one standard deviation (SD). The range thus contains 68% of a normal distribution of DRC factors. DRC (medium, low, high) must be multiplied by the dose the patient received to obtain the mean and the upper and lower limits, i.e., the so-called dose-related reference range.
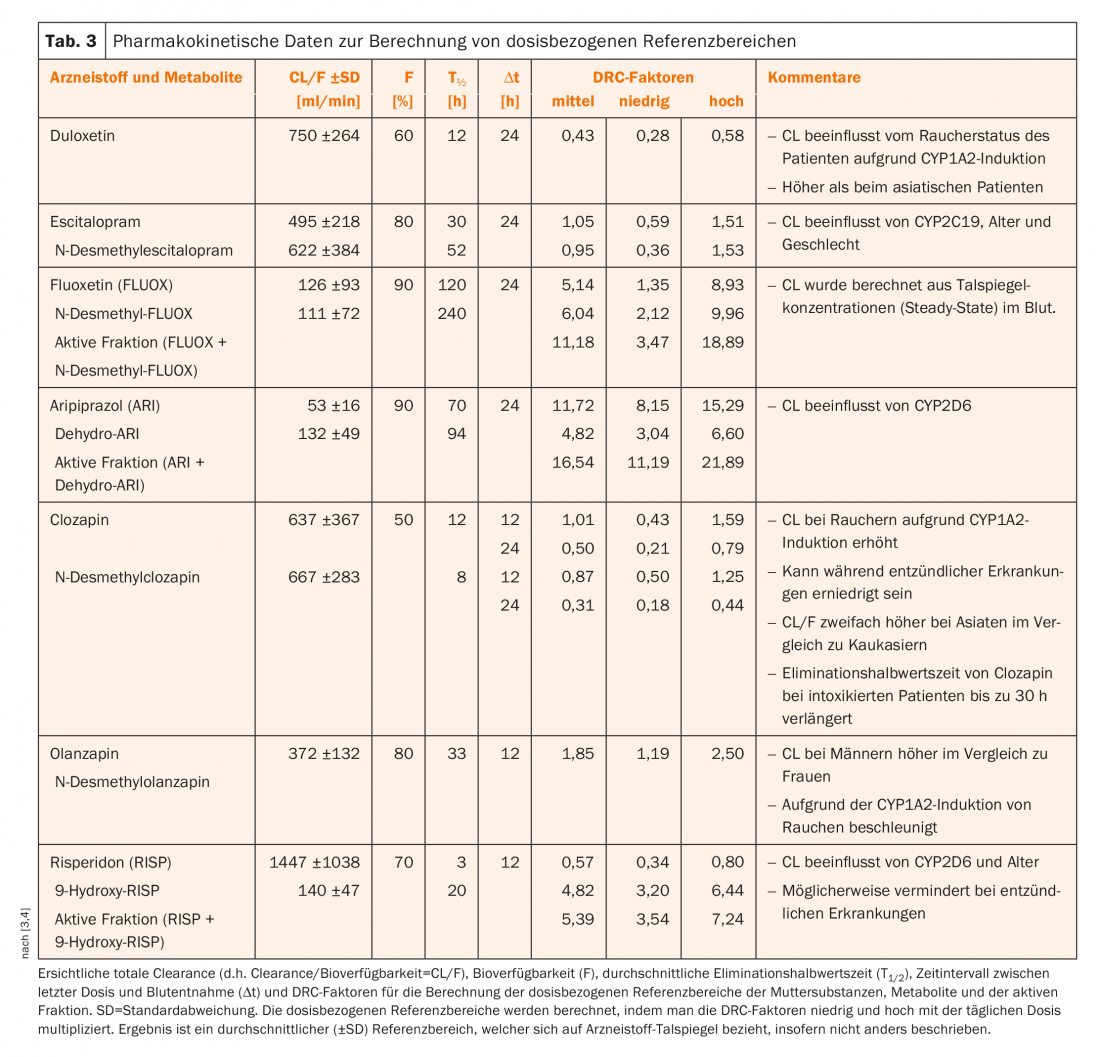
Patient A. was last treated with 40 mg/day olanzapine. Thus, the dose-related reference range calculated using the DRC factors is between 1.19 × 40 = 48 ng/mL (low) and 2.50 × 40 = 100 ng/mL (high). Thus, the measured blood level of olanzapine (118 ng/mL, 48 hours after discontinuation) is well above the normally expected drug concentration – especially when the laboratory calculated that the concentration must have been above 200 ng/mL at the time of the accident.
Interpretation of the findings
Interpretation of findings is an important stage within the TDM process to maximize the benefits associated with TDM. It is particularly challenging because it requires good theoretical knowledge of drug pharmacokinetics and pharmacodynamics, as illustrated by the clinical example.
In patient A, there are multiple causes for the increase in olanzapine levels:
- The dose increase
- CYP1A2, which is induced in smokers, is the main enzyme of olanzapine degradation. This induction largely ceased within a few days [6] after the patient stopped smoking [7], so slower clearance of olanzapine was to be expected.
- Possibly infection, as increases in immune mediators may result in inhibition of CYP1A2.
Supplementary is of importance: an inhibitory effect of CYP1A2 by infections has been reported so far for clozapine, but not yet for olanzapine. TDM clarified that the patient had probably suffered his accident because of olanzapine-induced delirium. Concentrations were in ranges reported with olanzapine depot in postinjection syndrome [8]. There was no evidence of suicidality. The patient was transferred to the psychiatric hospital after drug levels dropped below 100 ng/mL and then discharged.
On the way to personalized therapy
The AGNP TDM guideline aimed to summarize therapeutic and dose reference ranges, laboratory warning thresholds, indications, and levels of recommendation for the use of TDM for more than 154 neuropsychotropic drugs. Table 4 provides an incomplete overview of other topics discussed in the guideline. The Consensus Guideline for TDM in Neuropsychopharmacology contains a wealth of information based on the review of over 1350 references and consideration of the authors’ personal experience. Together with the practical advice, it enables appropriate application of TDM for optimal and personalized therapy of the psychiatric patient. Regarding the use of pharmacogenetic testing, reference is made to a critical review recently published in this journal [9]. Clearly, the best possible use of TDM in neuropsychiatry requires collaboration between the treating physician, the laboratory, and a professional specializing in pharmacokinetics.

Take-Home Messages
- The determination of psychotropic drugs in the blood (therapeutic drug monitoring, TDM) contributes to an optimal and personalized therapy of the psychiatric patient.
- The consensus guidelines of the AGNP TDM group are now available as a renewed and greatly expanded edition, again taking into account the combination of TDM and pharmacogenetic testing.
- This paper presents a brief introduction to these guidelines, the original version of which is freely available at www.agn.de.
Literature:
- Baumann P, et al: The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry 2004; 37(6): 243-265.
- Hiemke C, et al: AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 2011; 44(6): 195-235.
- Hiemke C, et al: Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry 2018; 51(1-2): 9-62.
- Hefner G, et al: Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: 2017 update. Psychopharmacotherapy 2017; 25(3): 92-140.
- Unterecker S, et al: Therapeutic drug monitoring in neuropsychopharmacology. Summary of the 2017 consensus guidelines of the TDM working group of the AGNP. Neurologist 2018.
- Faber MS, Fuhr U: Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clinical Pharmacology and Therapeutics 2004; 76(2): 178-184.
- Ring BJ, et al: Identification of the human cytochromes P450 responsible for the in vitro formation of the major oxidative metabolites of the antipsychotic agent olanzapine. J Pharmacol Exp Ther 1996; 276(2): 658-666.
- Sarangula SM, et al: Postinjection delirium/sedation syndrome with olanzapine depot injection. Indian J Psychol Med 2016; 38(4): 366-369.
- Baumann P, et al: Personalized therapy in psychotropic drugs: principles and practical guidance for pharmacogenetic testing. Info Neurol Psych 2017; 15(6): 21-30. The French version can be found at www.medizinonline.com/artikel/baumann_pharmacogenomique_therapie, as of 1/18/19.
InFo NEUROLOGY & PSYCHIATRY 2019; 17(1): 20-24.