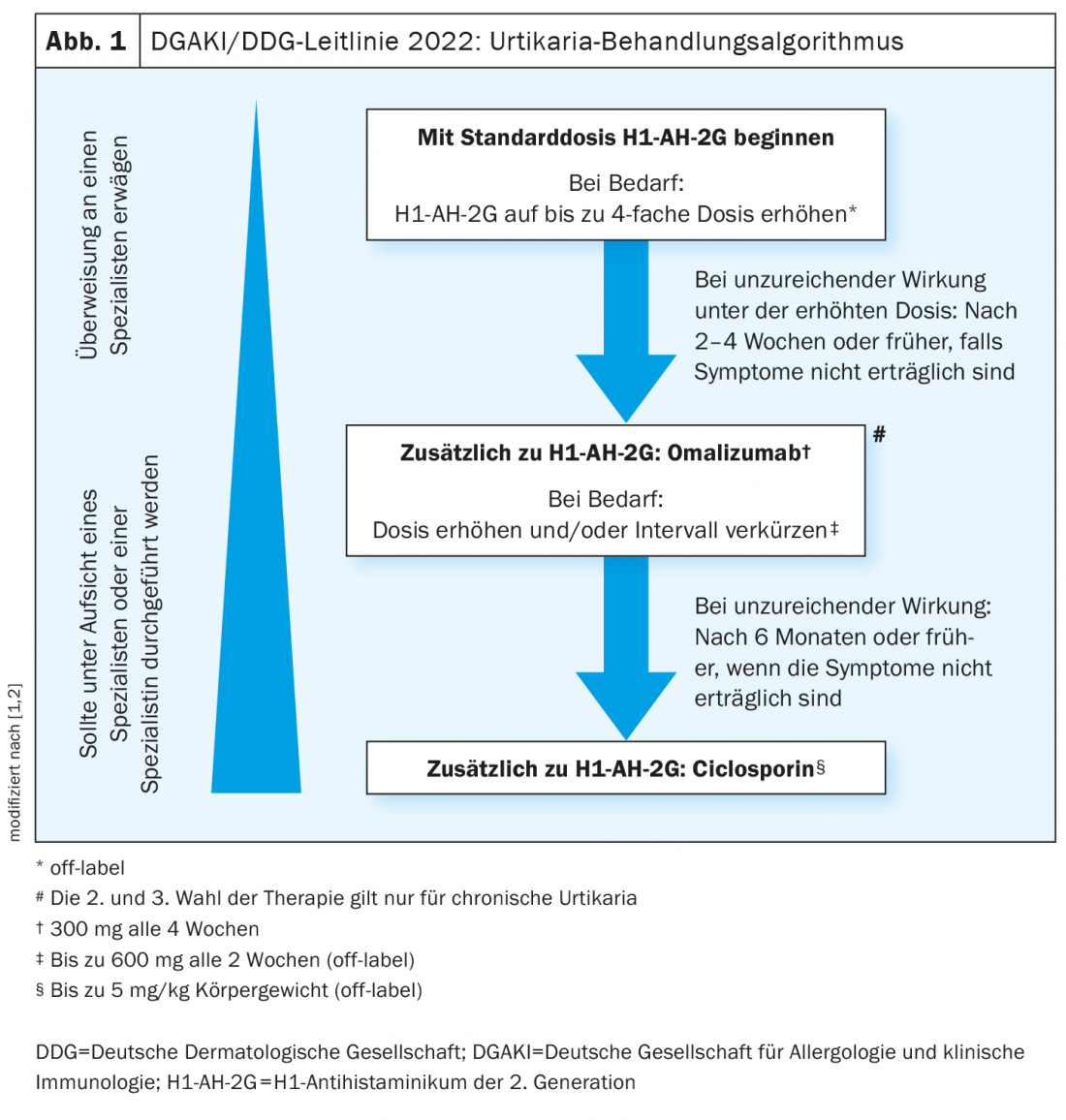
At the beginning of 2022, the new urticaria guideline of the German Society for Allergology and Clinical Immunology and the German Dermatological Society was published. Second-generation antihistamines and omalizumab as an add-on continue to act as the mainstays of treatment. But regarding dosage regimens, new recommendations have been included, based on findings of the international EAACI/GA²LEN expert group.
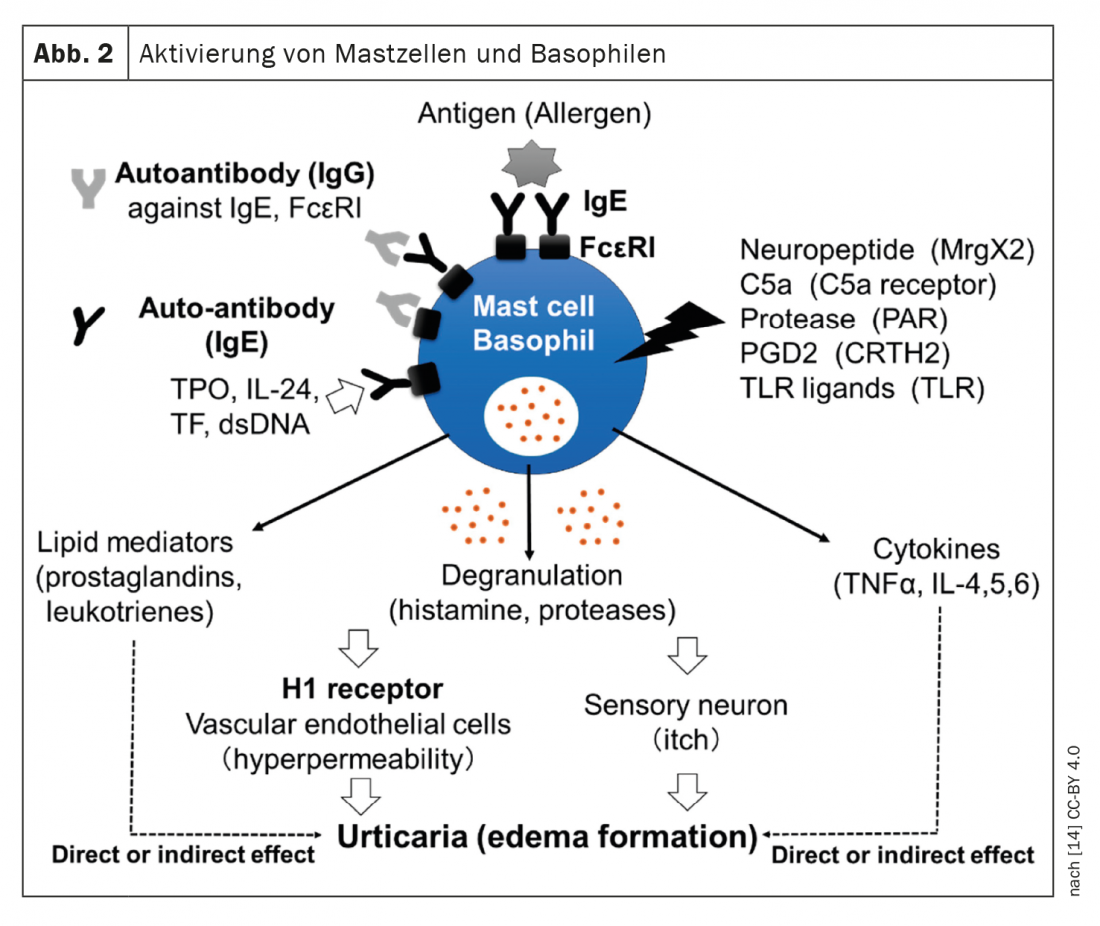
The updated S3 guideline is a German-language adaptation of the English-language EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline [1,2]. Prof. Dr. med. Torsten Zuberbier, Head of Allergy Consequence Research at the Clinic for Dermatology, Venereology and Allergology at Charité Universitätsmedizin Berlin, presented these at this year’s EEACI Annual Meeting [3]. Urticaria is a common mast cell-mediated disease characterized by the appearance of wheals and/or angioedema. Currently recommended treatment options involve mast cell mediators such as histamine or activators such as autoantibodies (Fig.1). While acute urticaria is self-limiting and usually does not require diagnostic work-up besides history for possible trigger factors, the diagnostic work-up in patients with chronic spontaneous urticaria (CSU) includes differential blood work, including CRP and/or erythrocyte sedimentation rate. In patients under specialist care, total IgE and IgG anti-TPO should also be collected. The reason for this is that patients with very low IgE usually do not respond well to omalizumab, whereas those with a positive IgG anti-TPO usually show a very good response to this monoclonal antibody, explained Prof. Zuberbier [3]. In individual patients, further diagnostic tests may be useful, according to the expert, who adds: “In inducible urticaria, we recommend looking only at the thresholds,” i.e., testing cold stimuli, etc. [3].
The overall therapeutic goal in all urticaria patients is to achieve complete symptom control.
Dose up 2nd generation H1 antihistamines, but do not mix them
Disease activity should be assessed at the initial and each subsequent examination. Validated questionnaires such as the Urticaria Activity Score (UAS) and the Angioedema Activity Score (AAS) can be used for this purpose. The UAS7 is based on the UAS (box). “For me personally, it is the most important diagnostic tool,” says Prof. Zuberbier [3]. These are very robust measured values, which also provide information on fluctuations over the course of one or more weeks. In this way, therapy-relevant correlations can be detected.

In order for patients to understand that strong and consistently effective treatment is needed, it is important to explain to them that even when the skin is free of symptoms, inflammatory activity may be present beneath the skin’s surface, the expert emphasized. The concept of “chronic minimal persistent inflammation” is known from other allergy-associated diseases and has also been confirmed in urticaria.
It is recommended that a 2nd-generation H1 antihistamine be used as first-line therapy for all types of urticaria [1,2] . These agents abolish the effect of histamine at the H1 receptor. According to the guideline, evidence-based effective substances approved in Switzerland are for example bilastine, cetirizine, desloratadine, fexofenadine, and levocetirizine [1,2], In patients with chronic urticaria, an increase of the dose up to four times the standard dose* is recommended as a second-line therapy option in case of lack of response. “Dosing up helps to reduce cytokine release,” explains Prof. Zuberbier [3]. On the other hand, the simultaneous use of various H1 antihistamines is not recommended. One of the reasons for this is that not only histamines but also other cytokines are involved, the speaker explained [3].
* off-label
Omalizumab as an add-on: Evaluating treatment response
In patients who do not show an adequate response to (up-dosed) antihistamine treatment after 2-4 weeks, adjunctive therapy with omalizumab is recommended [1,2]. If the standard dosage of 300 mg every 4 weeks is not effective, it has recently been advised to prescribe omalizumab in higher doses and/or at shorter intervals. Studies support the use of omalizumab at doses up to 600 mg and intervals of 2 weeks. There are numerous studies on the prediction of treatment response. Accordingly, low IgE levels at baseline correlate negatively with treatment response to omalizumab, but are positively correlated with response to ciclosporin [3,4]. That a dose increase may be useful in the absence of response to omalizumab is shown by the results of a multicenter observational study [5]. A subgroup of CSU patients who had a UAS7 >6 on omalizumab 300 mg every four weeks were up-titrated to 450 mg, and some were subsequently up-titrated to 600 mg, at dose intervals of four weeks each. 75% of patients on the up-dose treatment achieved a UAS7 ≤6, i.e., good symptom control.
As shown in a survey study conducted during the corona pandemic, trained patients can self-administer omalizumab at home [6]. 83% of respondents desired long-term continuation of therapy in the home setting. Concerns about home administration, such as injection errors or forgetting a dose, were very rarely mentioned.
Is omalizumab effective even after interruption of therapy?
Complete control implies flexible management of urticaria, according to Prof. Zuberbier [3]. For example, depending on the treatment outcome, omalizumab may be suspended or a dose increase may be considered. The OPTIMA study [7,8] demonstrated that the anti-IgE antibody is effective even after interruption of therapy. Adult CSU patients received treatment with omalizumab 150 mg or 300 mg as an add-on, in addition to H1 antihistamines, at dose intervals of 4 weeks. 115 of the total 314 CSU patients so treated achieved good symptom control (UAS7 ≤6) at week 24. However, an average of 44.5% of the 150-mg group and 50% of the 300-mg group relapsed 4.7 weeks after omalizumab discontinuation (UAS7 ≥16). After resumption of omalizumab therapy, 87.8% of affected individuals achieved renewed symptom control (UAS7 ≤6).
Ciclosporin A as “ultima ratio”.
In patients with chronic urticaria who do not respond to high-dose H1 antihistamines and omalizumab, the use of ciclosporin A (CSA) may be recommended in addition to antithistamine therapy. CSA has an immunosuppressive effect and a moderate, direct effect on the release of mast cell mediators. The efficacy of CSA in combination with a modified 2nd generation H1 antihistamine has been shown in placebo-controlled trials [9–11] in CSU, but this drug cannot be recommended as standard treatment due to a higher incidence of adverse effects [10].
Long-term or regular use of systemic glucocorticosteroids is not recommended in the guideline [1,2].
Congress: EEACI Annual Meeting
Literature:
- Zuberbier Z, et al: German S3 guideline on classification, diagnosis and therapy of urticaria, adapted from the international S3 guideline, 2022. AWMF guideline register (013-028).
- Zuberbier T, et al: The international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022; 77(3): 734-766.
- “Urticaria – the 2021 update on diagnosis and management,” Prof. Torsten Zuberbier, MD, EEACI Hybrid Congress, 1-3 July 2022.
- Ertas R, et al: The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 2018; 73(3): 705-712.
- Curto-Barredo L, et al: Omalizumab updosing allows disease activity control in patients with refractory chronic spontaneous urticaria. Br J Dermatol 2018; 179(1): 210-212.
- King C, et al: Rapid transition to home omalizumab treatment for chronic spontaneous urticaria during the COVID-19 pandemic: A patient perspective. World Allergy Organ J 2021; 14(10): 100587.
- Lynde C, et al: Omalizumab Retreatment of Patients with Chronic Idiopathic Urticaria/Chronic Spontaneous Urticaria Following Return of Symptoms: Primary Results of the OPTIMA Study EADV. Poster abstract presented at the Clinical Dermatology Conference 2017.
- Sussman G, et al: Omalizumab Re-Treatment and Step-Up in Patients with Chronic Spontaneous Urticaria: OPTIMA Trial. J Allergy Clin Immunol Pract 2020; 8(7): 2372-2378.e5.
- Grattan CE, et al: Randomized double-blind study of cyclos porin in chronic ‘idiopathic’ urticaria. Br J Dermatol 2000; 143(2): 365-372.
- Vena GA, et al: Cyclosporine in chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled trial. Journal of the American Academy of Dermatology. Oct 2006; 55(4): 705-709.
- Kulthanan K, et al: Cyclosporine for Chronic Spontaneous Urticaria: A Meta-Analysis and Systematic Review. J Allergy Clin Immunol Pract 2018; 6(2): 586-599.
- Mlynek A, et al: How to assess disease activity in patients with chronic urticaria? Allergy 2008;63(6): 777-780.
- Mathias SD, et al: Development of a daily diary for patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 2010;105(2): 142-148.
- Yanase Y, et al: The Role of Coagulation and Complement Factors for Mast Cell Activation in the Pathogenesis of Chronic Spontaneous Urticaria. Cells 2021; 10(7): 1759.
DERMATOLOGIE PRAXIS 2022; 32(4): 16-18 (published 8/22, ahead of print).