The results of a meta-analysis published in 2023 in the journal European Archives of Psychiatry and Clinical Neuroscience confirm that a proprietary lavender oil extract is an evidence-based anxiolytic herbal alternative to synthetic psychotropic drugs. Five randomized, placebo-controlled studies on generalized anxiety disorder (GAD), subsyndromal anxiety disorders (SSAD) and mixed anxiety and depression (MADD) were included.
Anxiety disorders can have a significant negative impact in various areas of life and are associated with a high burden of disease if not adequately treated [1–3]. Conventional synthetic psychotropic drugs such as selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs) and benzodiazepines are highly effective, but many patients are afraid of side effects such as dependence or sedation [4]. The lavender oil extract Silexan® obtained from the flowers of the medicinal lavender Lavandula angustifolia is one of the best-studied herbal active ingredients for the treatment of subsyndromal anxiety disorders, generalized anxiety disorder and mixed anxiety and depressive moods, with a total of over 14 clinical studies involving more than 2,200 patients and test subjects [5,6]. Studies show that Silexan® has no sedative effects and therefore does not impair the ability to drive; in addition, this phytopreparation is not addictive.
The lavender oil extract Silexan® exerts its anxiolytic effect by modulating the presynaptic calcium channels in the brain, which influences the voltage-gated calcium canal channel (VOCC ) in the central nervous system and subsequently also the release of neurotransmitters such as noradrenaline and glutamate [7].
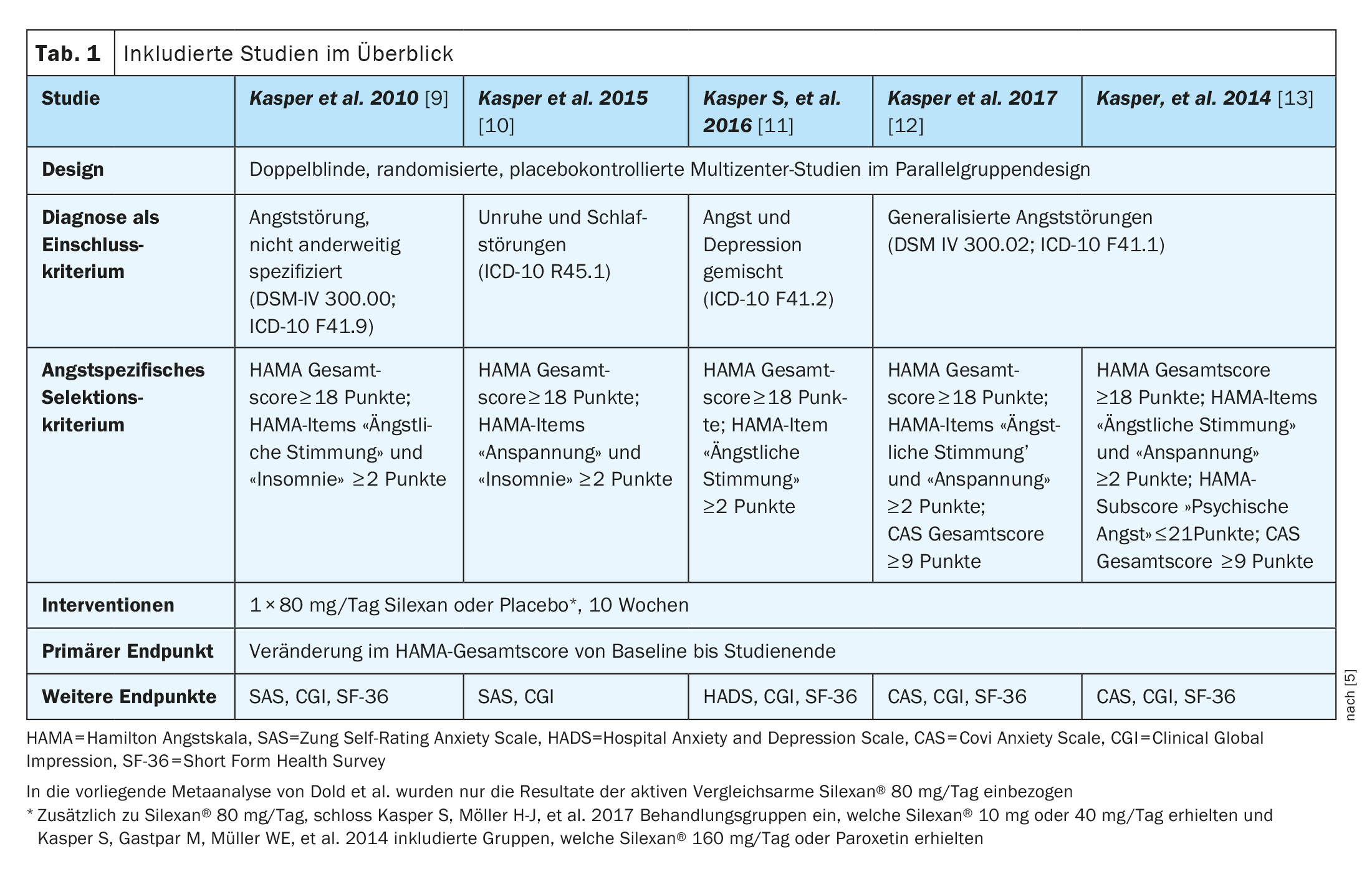
The meta-analysis by Dold et al. five randomized, double-blind, placebo-controlled clinical trials were included (Table 1): two studies each on generalized anxiety disorder (GAD) and subsyndromal anxiety disorder (SSAD) and one study on mixed anxiety and depressive disorder (MADD) [5]. In summary, it was confirmed that the lavender oil Silexan® (active ingredient of the drug Laitea®) [6] achieves significant anxiolytic effects in patients with GAD, SSAD or MADD and is well tolerated and safe. In each of the five studies, adult outpatients were randomized to Silexan 1×80 mg/day or placebo (n=1213). The primary endpoint was the Hamilton Anxiety Scale (HAMA, inclusion criterion HAMA ≥18), a questionnaire with which the severity of an anxiety disorder can be determined by external assessment. The secondary endpoints included the overall clinical impression (Clinical Global Impression, CGI) and the patient’s self-assessment of anxiety and quality of life (Short Form 36 Health Survey, SF-36).
Convincing risk-benefit profile
As a result, the patients in the Silexan® group showed a significant improvement in the overall HAMA score [6]. With regard to a ≥50% reduction in the HAMA total score, the ratio of the proportion of responders under Silexan® to the proportion of responders under placebo (responder rate ratio, RRR) was 1.34 in favor of Silexan® and the RRR regarding a large or very large improvement in the CGI was 1.51 in favor of lavender oil extract [5]. The proprietary lavender oil also proved to be superior to placebo in terms of self-assessment and the psychological and somatic parameters measured using SF-36. On the one hand, anxious and depressive moods as well as sleep disorders improved, and on the other hand, muscular and sensory symptoms were also alleviated. The undesirable side effects of Silexan® were at placebo level. The number needed to treat (NNT) for Silexan® was 5, which corresponds to the NNT value of SSRIs for anxiety disorders [8].
In summary, this meta-analysis confirmed the significant and clinically important anxiolytic efficacy of Silexan®.
Literature:
- Bandelow B, Michaelis S, Wedekind D: Treatment of anxiety disorders. Dial Clin Neurosci 2017; 19: 93-107.
- Bandelow B, et al: The German Guidelines for the treatment of anxiety disorders: first revision. Eur Arch Psychiatry Clin Neurosci 2021; 1-12.
- Kasper S: Anxiety disorders: under-diagnosed and insufficiently treated. Int J Psychiatry Clin Pract 2006; 10: 3-9.
- Sartori SB, Singewald N: Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol Ther 2019; 204: 107402. doi: 10.1016/j.pharmthera.2019.107402.
- Dold M, Bartova L, Volz HP, Seifritz E, Möller HJ, Schläfke S, Kasper S: Efficacy of Silexan in patients with anxiety disorders: a meta-analysis of randomized, placebo-controlled trials. Eur Arch Psychiatry Clin Neurosci 2023; 273(7): 1615-1628.
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch/accept.aspx,(last accessed 07.02.2024)
- Kasper S, et al.: Silexan in anxiety disorders: Clinical data and pharmacological background. World J Biol Psychiatry 2018; 19: 412–420.
- Bandelow B et al: Meta-analysis of randomized controlled comparisons of psychopharmacological and psychological treatments for anxiety disorder; World J Biol Psychiatry 2007; 8(3): 175-187.
- Kasper S, Gastpar M, et al. Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’ anxiety disorder: a randomized, double-blind, placebo controlled trial. Int Clin Psychopharmacol 2010; 25: 277-287.
- Kasper S, Anghelescu I, Dienel A: Efficacy of orally administered Silexan in patients with anxiety-related restlessness and disturbed sleep – a randomized, placebo-controlled trial. Eur Neuropsychopharmacol 2015; 25: 1960-1967.
- Kasper S, Volz H-P, Dienel A, Schläfke S: Efficacy of Silexan in mixed anxiety-depression – a randomized, placebo-controlled trial. Eur Neuropsychopharmacol 2016; 26: 331-340.
- Kasper S, Möller H-J, Volz H-P, et al: Silexan in generalized anxiety disorder: investigation of the therapeutic dosage range in a pooled data set. Int Clin Psychopharmacol 2017; 32: 195-204.
- Kasper S, Gastpar M, Müller WE, et al: Lavender oil preparation Silexan is effective in generalized anxiety disorder – a randomized, double-blind comparison to placebo and paroxetine. Int J Neuropsychopharmacol 2014; 17: 859-869.
FAMILY PHYSICIAN PRACTICE 2024; 19(2): 24-25