In a retrospective study of 30 patients with moderate to severe plaque psoriasis, the use of the IL-23 inhibitor tildrakizumab was shown to be effective and safe in clinical practice. Of note, more than half of the patients had psoriasis in hard-to-treat sites. The study population included biologics-naive and biologics-experienced patients. The results were published in the Journal of the European Academy of Dermatology and Venereology.
Patients with moderate to severe plaque psoriasis (BSA$) ≥10%, PGA** score ≥3, and PASI# score ≥12) in whom at least two conventional treatment modalities, including systemic therapeutics or phototherapy, had not proven to be effective were included in the study [1,2]. Patients with a PASI score <10 were also included in the study if they had psoriasis lesions in difficult-to-treat locations such as the hands, face, scalp, or genital areas. This classification is consistent with the upgrade criteria of the current S3 guideline [3] .
$
Body surface area involvement
** Physician Global Assessment
# PASI=Psoriasis Area and Severity Index
| The study authors point out that nearly half of the patients (46.7%) who had previously been treated with a biologic achieved a comparable response to that seen in biologic-naïve patients [1]. This was evident from the PASI response and multivariate analyses and confirmed results from previous studies [1,4,5]. |
Patients with long-standing psoriasis and concomitant diseases
The mean age of the study participants was 46.3 ± 13.3 years and the mean disease duration was 14.7 ± 7.3 years. The majority of patients (70%) were men [1]. The included psoriasis patients had no obesity, and the most common comorbidities were hypertension and hypercholesterolemia. More than half of the participants (56.7%) had psoriasis in difficult-to-treat localizations, the most common being nail involvement (23.3%) and scalp involvement (16.7%). In total, 28 of the 30 patients had already undergone at least one treatment attempt with a systemic therapeutic: ciclosporin (n=16), acitretin (n=12), and methotrexate (n=12). 46.7% of patients had been treated at some time in advance with at least one of the following biologics: anti-TNF-α (n = 10), anti-IL17, (n = 5), anti-IL23, or anti-IL12/23 (n = 6). At baseline, all patients tested negative for hepatitis B and C, as well as HIV and tuberculosis.
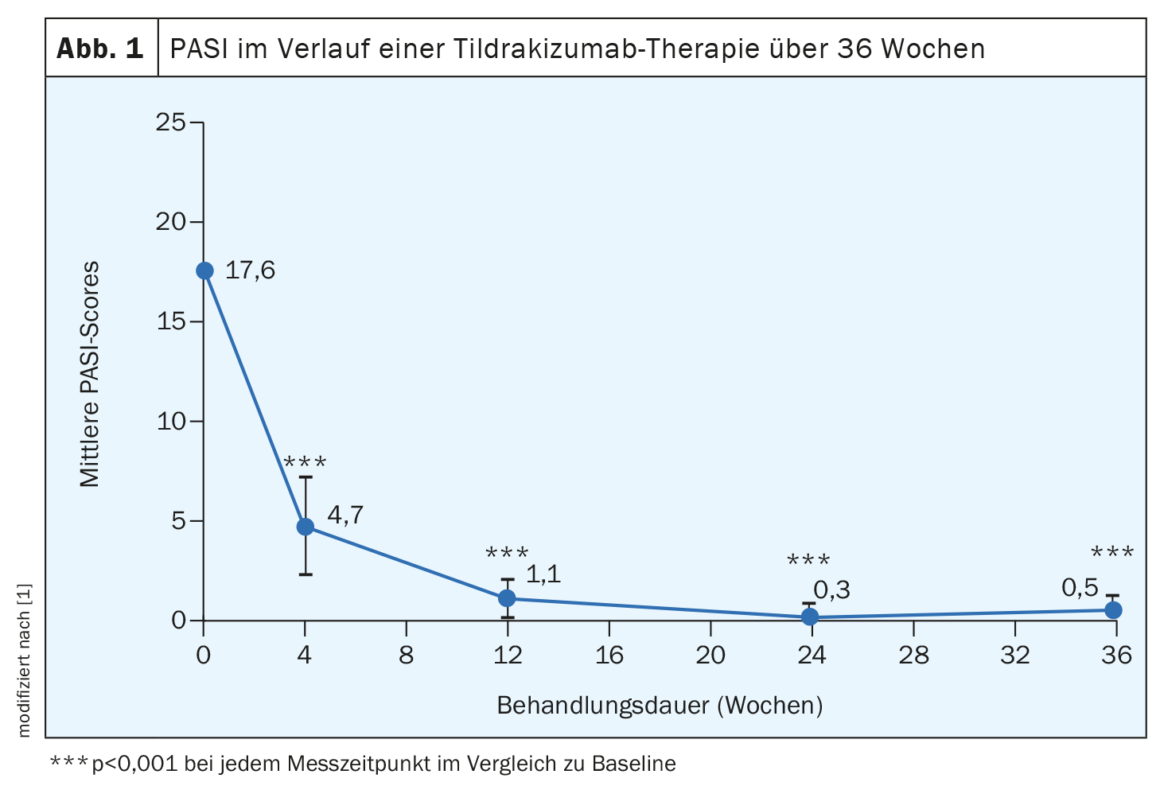
PASI values and other parameters improved strongly during the course
The dosing regimen usually recommended for tildrakizumab was used: 100 mg tildrakizumab (s.c.) at weeks 0 and 4 and every 12 weeks thereafter [1]. Clinical efficacy was assessed by PASI response at weeks 4, 12, 24, and 36. In addition, PGA, health-related quality of life, and the DLQI& were recorded. During the course of tildrakizumab treatment, the PASI score decreased significantly from 17.6 ± 4.7 at baseline to 4.7 ± 2.5 at week 4 and was 0.5 ± 0.7 at week 36 (Fig. 1). A PASI score ≤3 was achieved by 86.7% of patients at week 12 and by all participants at weeks 24 and 36. PASI 75, 90, and 100 response rates at week 36 were 100%, 96.7%, and 60%, respectively. PGA decreased from 2.8 ± 0.6 at baseline to 1 ± 0.2 at week 4 and was around ~0 in the following weeks. Mean DLQI scores were 13.8 ± 2.9 at baseline and decreased to 3.6 ± 1.6 at week 4 and 0 at week 36, respectively. Patient satisfaction also improved significantly over the course of treatment.
& DLQI=Dermatological Quality of Life Index
Tildrakizumab (Ilumetri®) is one of the three interleukin (IL)-23p19 antagonists currently approved in Switzerland for the treatment of plaque psoriasis [6].
Literature:
- Gambardella A, et al: Treatment of moderate-to-severe plaque psoriasis with tildrakizumab in the real-life setting. JDDG 2023; 2(1): 52-58.
- Gisondi P, et al: Italian guidelines on the systemic treatments of moderate-to-severe plaque psoriasis. JEADV 2017; 31(5): 774-790.
- Nast A et al. German S3 guideline for the therapy of psoriasis vulgaris, adapted by EuroGuiDerm – Part 1: Therapy goals and therapy recommendations. JDDG 2021, https://register.awmf.org,(last accessed Mar. 10, 2023).
- Galluzzo M, et al: Efficacy of tildrakizumab for the treatment of difficult-to-treat areas: scalp, nail, palmoplantar and genital psoriasis. J Clin Med 2022; 11(9): 2631.
- Poulin Y, et al: Efficacy of tildrakizumab by patient demographic and disease characteris-tics across a phase 2b and 2 phase 3 trials in patients with moderate-to-severe chronic plaque psoriasis. JEADV 2020; 34(7): 1500-1509.
- Drug Information, www.swissmedicinfo.ch,(last accessed Mar. 10, 2023).
DERMATOLOGY PRACTICE 2023; 33(2): 30