In patients with refractory arterial hypertension, a detailed history, physical examination, and 24-h ambulatory blood pressure measurement should be performed, as well as laboratory analyses of serum electrolytes, glucose, and renal retention parameters, and urine diagnostics with protein determination and sodium urine excretion. Catheter-based interventional renal sympathetic denervation (RDN) is a potent new interventional procedure with few complications for patients with proven resistance to therapy. In this procedure, an ablation catheter is inserted into the renal arteries via a femoral approach under X-ray fluoroscopy, through which ablation energy (e.g., radiofrequency current or ultrasound) can subsequently be delivered. This leads to obliteration of the sympathetic nerve fibers located in the adventitia. Contraindications to RDN include hypertension of secondary etiology, inappropriate renal artery anatomy, and GFR <45 ml/min per 1.73 m². The extent to which RDN is also suitable for the treatment of other diseases with increased sympathetic activity needs to be investigated in further studies.
Approximately 5-15% of all patients with hypertension have refractory arterial hypertension [1–3]. It is defined as a non-guideline blood pressure setting (>140/90 mmHg in general, >130-139/80-85 mmHg in patients with diabetes mellitus, >130/80 mmHg in chronic kidney disease) despite continuous use of triple antihypertensive therapy including a diuretic in an appropriate combination [2]. In the pathophysiology of the disease, the overactivity of the autonomic nervous system, due to a dysbalance between sympathetic and parasympathetic activity, is of paramount importance [4]. An increase in efferent sympathetic activity in the kidney results in increased renin secretion, increased sodium retention (proximal tubule), and decreased renal perfusion [4]. The purpose of this paper is to provide an overview of the treatment of refractory hypertension using interventional renal sympathetic denervation (RDN).
Patient selection
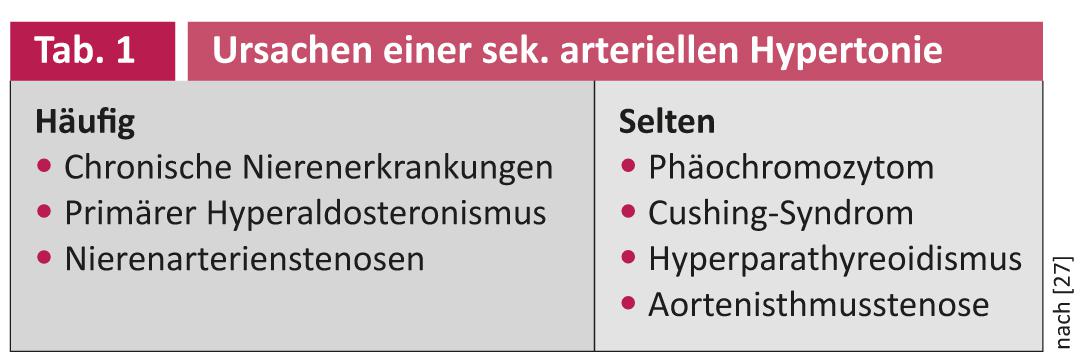
In principle, in addition to drug therapy, lifestyle-modifying measures are the basis of any antihypertensive treatment. A distinction must be made between true resistance to therapy and pseudoresistance, for example, due to a lack of drug adherence or situationally elevated blood pressure values in the sense of white coat hypertension [2]. In up to 20% of cases, secondary causes of hypertension underlie hypertension in patients with refractory hypertension (Table 1) [2]. Potentially reversible causes also include suboptimal antihypertensive therapy and hypertensive side effects of other medications (e.g., NSAIDs, cortisone). In addition to a detailed medical history, physical examination, and 24-hour ambulatory blood pressure measurement, laboratory analyses of serum electrolytes, glucose, and renal retention parameters as well as urine diagnostics with protein determination and sodium urine excretion should be an integral part of the diagnosis in patients with refractory arterial hypertension [5]. Screening for primary hyperaldosteronism is performed by determining the aldosterone-renin ratio. It is essential to pay attention to possible interactions with antihypertensive substances. If there is evidence of primary hyperaldosteronism (aldosterone-renin ratio >50), further imaging and side-separated adrenal vein blood sampling are recommended. Patients with episodic, crisis increases in blood pressure should be evaluated for the presence of pheochromocytoma. In addition, ultrasonography of the renal arteries is recommended to exclude atherosclerotic renal artery stenosis or fibromuscular dysplasia.
Contraindications
The current contraindications to RDN are hypertension of secondary etiology (except obstructive sleep apnea syndrome), inappropriate renal artery anatomy (diameter <4 mm, length <20 mm; fibromuscular dysplasia; significant renal artery stenosis), and GFR <45 ml/min per 1.73 m² [5]. A recently published pilot study (n=15) demonstrated that RDN can be safely and effectively performed even in patients with moderate to severe chronic kidney disease (mean GFR 31 ml/min per 1.73 m²) [6]. However, for the time being, the treatment of patients with higher-grade renal insufficiency should only be carried out in experienced centers and in the context of clinical trials.
Procedure
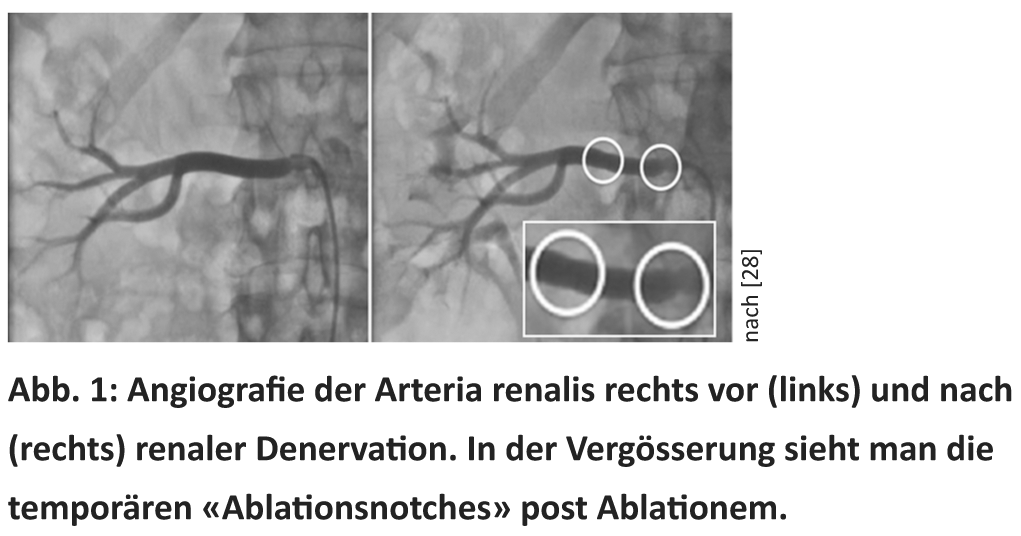
As early as the 1950s, surgical splanchniectomy was considered a backup procedure for the treatment of severe hypertension [7]. Radical transection of sympathetic nerve fibers has resulted in blood pressure reductions of up to 70 mmHg. However, the procedure very often resulted in serious complications such as massive dizziness, syncope, incontinence, and erectile dysfunction [7]. Recently, it has become possible to specifically obliterate renal sympathetic fibers by a minimally invasive catheter-based procedure. Currently, six CE-certified catheter systems are available for RDN (Medtronic® Symplicity, St Jude® EnligHTN, Vessix® The V2, Covidien® OneShot, Terumo Iberis and Recor® Paradies). For this purpose, an ablation catheter is inserted into the renal arteries via a femoral approach under X-ray fluoroscopy, through which ablation energy (e.g., radiofrequency current or ultrasound) can subsequently be delivered (Fig. 1). This leads to obliteration of the sympathetic nerve fibers located in the adventitia [8]. The procedure takes about 30-45 minutes, depending on the device, and is performed on both kidneys. Since sympathetic fibers are accompanied by C pain fibers, pain occurs briefly and only for the duration of energy delivery (30-120 seconds), so analgesia with opiates and sedatives is necessary for this moment [5]. Following the procedure, continuous follow-up of patients is recommended, usually every three to six months in the first year after the procedure, and later once a year [5].
Security
The procedure is considered low risk and comparable to cardiac catheterization. The procedure could be performed without complications in 201 of the 206 (98%) patients systematically enrolled in the pivotal studies. Four patients (1.9%) experienced postinterventional pseudoaneurysms of the femoral artery (prevalence with other interventions 0.8-2.2% [9]), all of which were treated conservatively. Major vascular injuries such as arterial dissection, aneurysm, or development of renal artery stenosis (<1%) are extremely rare [10, 11]. Similarly, after renal denervation, there was no evidence of orthostatic dysfunction, electrolyte disturbances, chronotropic incompetence [12] or adverse effect on renal function [11].
Clinical studies
In the multicenter proof-of-concept Symplicity HTN-1 trial, patients (n=45) on therapy with an average of 4.7 antihypertensive agents had a blood pressure of 177/101 mmHg before RDN [13]. Already after one month, a significant blood pressure reduction of 14/10 mmHg (p=0.026) was documented in the treatment group. This effect increased continuously over the follow-up period and was -27/-17 mmHg (p=0.026) after twelve months with unchanged antihypertensive medication. No rebound in blood pressure was seen during the study period or in the currently published extended follow-up over a 36-month period (Fig. 2) [14, 15]. This makes functional regeneration unlikely, so a longer-term effect can be assumed. The reduction in sympathetic activity by RDN was confirmed by a significant 47% decrease in renal norepinephrine release (n=10) and correlated with a reduction in blood pressure (-22/-12 mmHg) after six months [13].

In the Symplicity HTN-2 randomized controlled trial (n=106), mean arterial pressure was 178/96 mmHg despite use of a mean of 5.3 antihypertensive medications [16]. Six months after RDN, there was a significant reduction in blood pressure by 32/12 mmHg (p<0.0001), whereas blood pressure remained unchanged in the control group. By lowering blood pressure, a reduction in the medication or dose taken was achieved in 20% of patients. In 84% of patients, RDN resulted in a reduction in systolic blood pressure of at least 10 mmHg at six months. High systolic blood pressure at the time of study (p<0.001) and use of centrally acting sympatholytics (p=0.018) were independent predictors of significant blood pressure reduction [15].
In clinical trials conducted to date, the non-response rate has ranged from 8% to 17% [17]. Predictors of lack of response to treatment have not been identified to date. Long-term blood pressure measurement was available for 20 patients in the denervation group. The blood pressure reduction after six months was 11/7 mmHg (p=0.007/0.014), whereas there were no significant changes in the control group [18]. This year also saw the first collection of data on patients with milder forms of refractory hypertension with systolic blood pressure between 140 and 160 mmHg [19]. In this group, RDN reduced blood pressure by 13/5 mmHg (p<0.001) at six months.
Currently, the multicenter, single-blind, randomized, placebo-controlled Symplicity HTN-3 trial (NCT01418261) is enrolling patients in the United States. That study will hopefully answer the question of what the placebo effect contributes to blood pressure reduction after RDN. Clinical registries and continuous follow-up of treated patients are necessary to conclusively assess the long-term effects and safety of the procedure. To this end, both national (German Renal Denervation [GREAT] Registry) and international (Symplicity Global Registry; NCT01534299) registries are in the pipeline.
Pleiotropic effects
Initial study results suggest that glucose metabolism may also improve after RDN in patients with refractory hypertension (Fig. 3) [20].
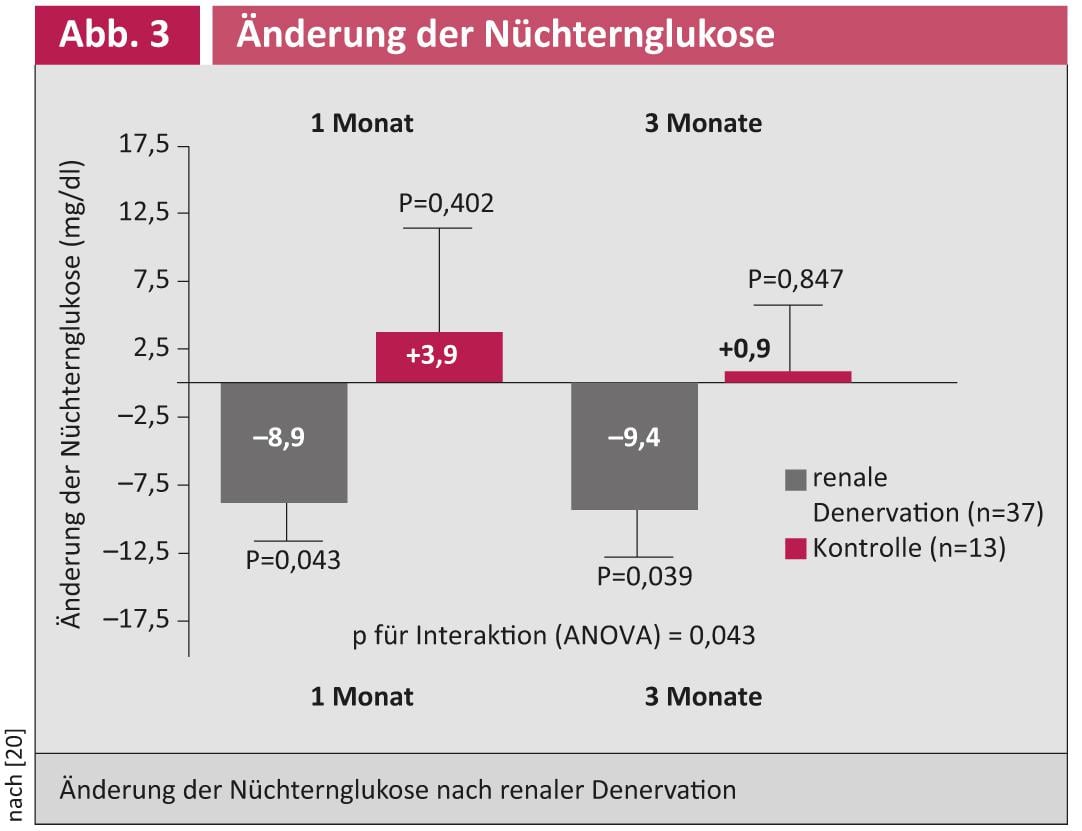
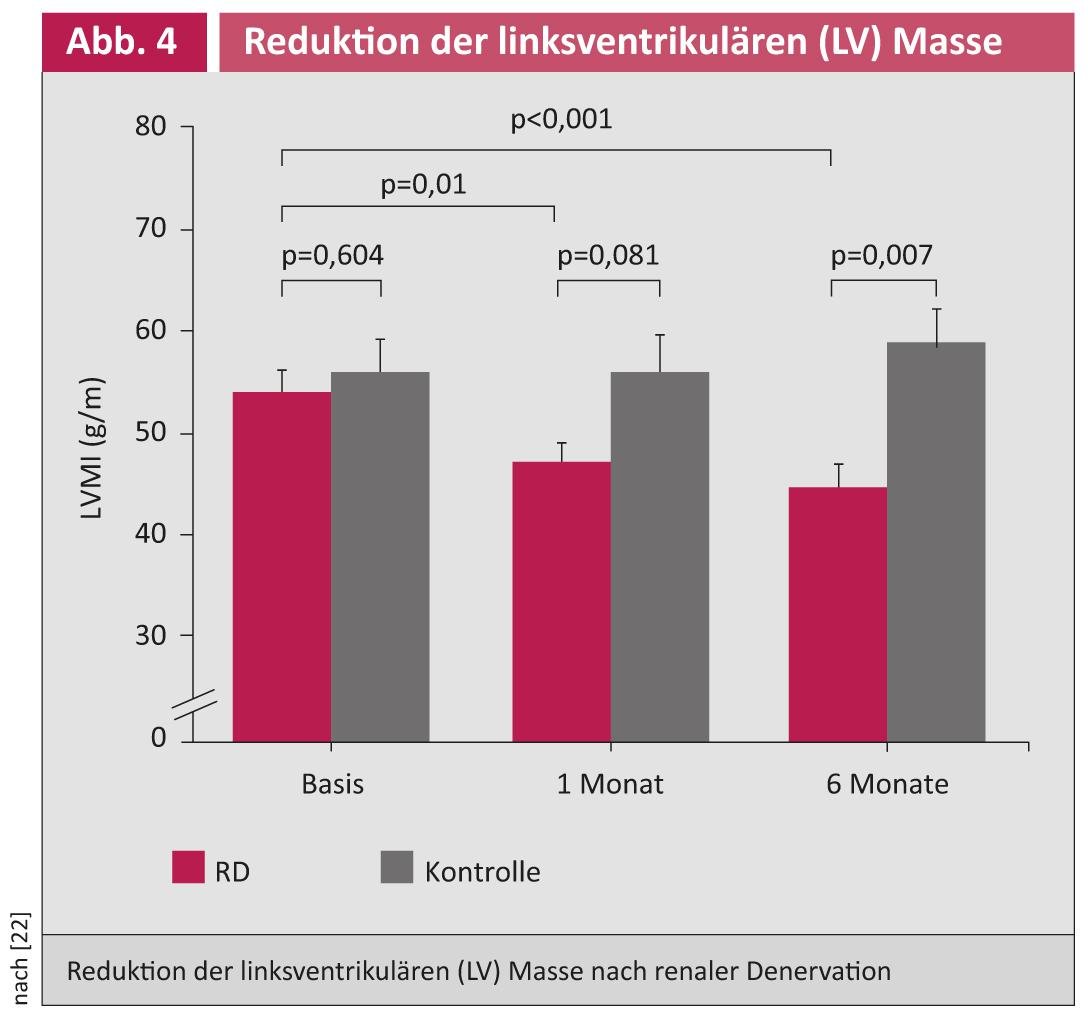
In a pilot study, blood pressure reduction, glucose metabolism improvement, and apnea/hypopnea index reduction were demonstrated after RDN in patients with refractory hypertension and obstructive sleep apnea syndrome [21]. An echo substudy demonstrated that RDN can lead to a reduction in left ventricular mass (especially in left ventricular hypertrophy) and an improvement in diastolic function (Fig. 4) [22].
Furthermore, first positive effects have also been shown in patients with chronic heart failure [23, 24] in relation to blood pressure control in condition after aortic dissection type B [25] as well as chronic renal failure [26].
Literature:
- Lim SS, et al: A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380 (9859): 2224-2260 doi:10.1016/S0140-6736(12)61766-8.
- Calhoun DA, et al: Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25): e510-526 doi:10.1161/CIRCULATIONAHA.108.189141.
- Daugherty SL, et al: (2012) Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; 125(13): 1635-1642 doi:10.1161/CIRCULATIONAHA.111.068064.
- Sobotka PA, et al: Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100 (12): 1049-1057 doi:10.1007/s00392-011-0335-y.
- Mahfoud F, et al: Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013 doi:10.1093/eurheartj/eht154.
- Herring D, et al: Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; 23(7): 1250-1257 doi:10.1681/ASN.2011111062.
- Smithwick RH, Thompson JE: Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152 (16): 1501-1504.
- Atherton DS, et al: Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat 2012; 25 (5): 628-633 doi:10.1002/ca.21280.
- Lenartova M, Tak T: Latrogenic pseudoaneurysm of femoral artery: case report and literature review. Clin Med Res 2003; 1(3): 243-247.
- Vonend O, et al: Secondary rise in blood pressure after renal denervation. The Lancet 2012; 380(9843): 778 doi:10.1016/s0140-6736(12)61145-3.
- Mahfoud F, et al: Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 2012; 60(2): 419-424 doi:10.1161/HYPERTENSIONAHA.112.193870.
- Ukena C, et al: Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58 (11): 1176-1182 doi:10.1016/j.jacc.2011.05.036.
- Krum H, et al: Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373(9671): 1275-1281 doi:10.1016/S0140-6736(09)60566-3.
- Esler MD, et al: Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 2012; 126(25): 2976-2982 doi:10.1161/CIRCULATIONAHA.112.130880.
- Symplicity HTNI: Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57(5): 911-917 doi:10.1161/HYPERTENSIONAHA.110.163014.
- Esler MD, et al: Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376(9756): 1903-1909 doi:10.1016/S0140-6736(10)62039-9.
- Ukena C, et al: Response and non-response to renal denervation: who is the ideal candidate? EuroIntervention 2013; 9 Suppl R: R54-57 doi:DOI: 10.4244/EIJV9SRA10.
- Mahfoud F, et al: Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation 2013; 128(2): 132-140 doi:10.1161/CIRCULATIONAHA.112.000949.
- Kaltenbach B, et al: Renal sympathetic denervation as second-line therapy in mild resistant hypertension: A pilot study. Catheter Cardiovasc Interv 2013; 81(2): 335-339 doi:10.1002/ccd.24557.
- Mahfoud F, et al: Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123(18): 1940-1946 doi:10.1161/CIRCULATIONAHA.110.991869.
- Witkowski A, et al: Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58(4): 559-565 doi:10.1161/HYPERTENSIONAHA.111.173799.
- Brandt MC, et al: Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59(10): 901-909 doi:10.1016/j.jacc.2011.11.034.
- Davies JE, et al: First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol 2012 doi:10.1016/j.ijcard.2012.09.019
- Ukena C, et al: Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol 2012; 101(1): 63-67 doi:10.1007/s00392-011-0365-5.
- Ewen S, et al: First-in-human experience: percutaneous renal denervation through a false lumen fenestration in aortic dissection type B. EuroIntervention 2013; 8(9): 1110 doi:10.4244/eijv8i9a170.
- Ewen S, et al: The sympathetic nervous system in chronic kidney disease. Curr Hypertens Rep 2013 doi:10.1007/s11906-013-0365-0.
- Mahfoud F, et al: Treatment strategies for resistant arterial hypertension. Dtsch Arztebl Int 2011; 108(43): 725-731 doi:10.3238/arztebl.2011.0725.
- Ewen S, et al: Percutaneous renal denervation: new treatment option for resistant hypertension and more? Heart 2013 (Epub before printing).
- Krum, et al: J Am Coll Cardiol 2012; 59: E1704.