Both protein kinase inhibitors and ipilimumab/nivolumab combined have better efficacy than their respective monotherapies in patients with non-resectable melanoma. This has been known for some time. An interesting new approach is the triple therapy of BRAF/MEK inhibition and a checkpoint inhibitor, as shown by recently published data from the IMspire150 trial.
The addition of the PDL1 inhibitor atezolizumab to vemurafenib/cobimetinib combination therapy in the initial phase of treatment resulted in improved outcomes in patients with newly diagnosed BRAF V600E/K-mutated advanced melanoma in a placebo comparison. Prof. Dr. med. Lucie Heinzerling from the University Hospital Erlangen (D) spoke about this and other current findings in this field during the dermato-oncology session of the 3-country refresher “Immuno-oncologics and Targeted Therapies” in Hofheim (D) [1].
IMspire150 study: promising results
IMspire150 [2] is an international study of 514 melanoma patients randomized in a 1:1 ratio to the triple therapy treatment arm and the BRAF/MEK control condition, respectively. Subjects received vemurafenib 960 mg twice daily and cobitimetinib 60 mg daily on days 1 through 21 within a four-week treatment cycle. In the study condition, atezolizumab 840 mg was administered on days 1 and 15; in the control arm, study participants received placebo instead. All subjects had non-resectable melanoma with BRAF V600 mutation, 94% of patients had metastases. The mean age of the study participants was 54 years.
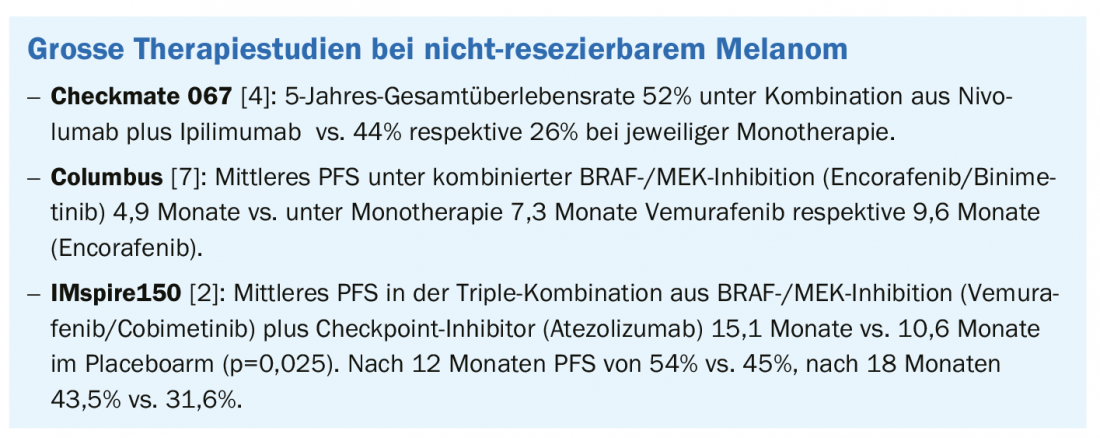
The triple combination was superior in terms of progression-free survival (PFS) and resulted in more durable responses, according to the initial results of the phase III trial presented at the 2020 ASCO* meeting [3] and published in the Lancet [2]. The atezolizumab condition achieved a significantly higher PFS of 15.1 months on average, compared with 10.6 months in the placebo condition. At 12 months, PFS was 54% vs. 45% in the control condition, and at 18 months, 43.5% vs. 31.6%. In conclusion, based on this interim analysis, the treatment arm with atezolizumab proved superior to the control condition.
* ASCO=American Association for Cancer Research
Combining advantages of BRAF/MEK inhibition and checkpoint inhibitors
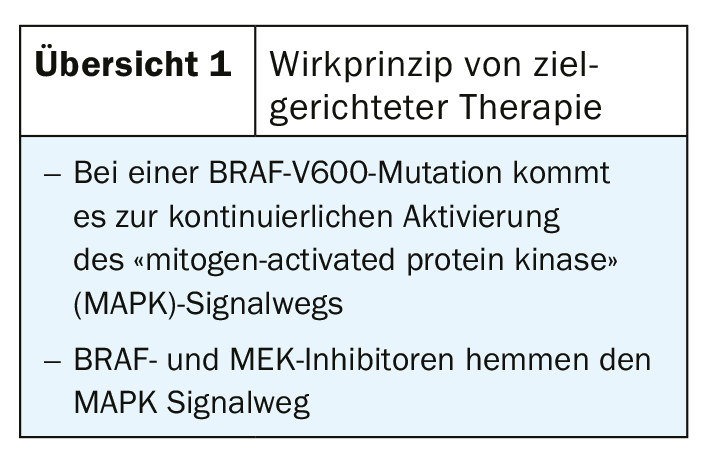
BRAF/MEK inhibition (Overview 1) results in a high response rate in the short term and checkpoint inhibitors (Overview 2) have a slightly lower response but are more durable. By combining these drug classes, the respective limitations can be compensated, resulting in higher and more durable response rates. In the CheckMate 067 trial [4], the 5-year overall survival rate in patients with metastatic melanoma was 52% with nivolumab plus ipilimumab combination, 44% with nivolumab therapy alone, and 26% with ipilimumab monotherapy. Progression-free in the nivolumab/ipilimumab cohort was 36% of patients after 5 years. These are impressive long-term data, which show that the market approval of checkpoint inhibitors has sustainably improved the options for melanoma treatment. In Switzerland, the following checkpoint inhibitors are approved for melanoma treatment [5,6]: Ipiliumumab (Yervoy®), which belongs to CTLA-4, and the PD1 inhibitors nivolumab (Opdivo®) and pembrolizumab (Keytruda®). In targeted therapy, a new BRAF/MEK inhibitor combination therapy was approved in Switzerland at the end of 2019 for adult patients with non-resectable or metastatic melanoma with a BRAFV600 mutation: encorafenib (BRAFTOVI®)/binimetinib (MEKTOVI®) [5,6]. The approval is based on the Columbus trial, in which the primary endpoint of progression-free survival as well as several secondary endpoints were met [7]. This was an open-label, active-controlled phase III trial in 577 patients with locally advanced, unresectable, or metastatic malignant melanoma with BRAFV600 mutation (subtype V600 E or K) [7]. With a median PFS on combination targeted therapy of 14.9 months, encorafenib plus binimetinib was significantly superior to monotherapy with vemurafenib (7.3 months) and encorafenib, respectively (9.6 months). In addition to encorafenib/binimetinib, dabrafenib/trametinib and vemurafenib/cobimetinib are also available as BRAF/MEK inhibitor combination therapies [1].

There is also new evidence on the role of the microbiome as a marker of treatment efficacy. Analysis of microbiome from stool samples as well as oral samples from melanoma patients prior to implementation of anti-PD1 therapy indicate that greater diversity of gut flora is associated with better response [11]. According to the speaker, further investigations are currently underway. In addition to predicting treatment response, the management of adverse effects currently remains a major challenge. Checkpoint inhibitors induce side effects in all organ systems [8] that are recorded in the Serio Registry (www.serio-registry.org). The reports were from 21 centers in 5 countries, including CH and D [9]. BRAF/MEK inhibition is also associated with side effect risks, and the most common adverse effects include nausea, diarrhea, and vomiting. However, BRAF inhibitor-induced skin reactions were found to be less in encorafenib/binimetinib combination therapy than in monotherapy [10].
Source: FOMF 3-Country Refresher
Literature:
- Heinzerling L: Dermato-Oncology Session, 3 Country Refresher “Immuno-oncologics and Targeted Therapies”, Forum for Continuing Medical Education. Prof. Lucie Heinzerling, MD, Hofheim (D), June 19, 2020.
- Gutzmer R, et al: Atezolizumab, Vemurafenib, and Cobimetinib as First-Line Treatment for Unresectable Advanced BRAF V600 Mutation-Positive Melanoma (IMspire150): Primary Analysis of the Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2020; 395(10240): 1835-1844.
- McArthur GA, Stroyakovskiy D, Gogas H, et al: Evaluation of atezolizumab, cobimetinib, and vemurafenib in previously untreated patients with BRAF V600 mutation-positive advanced melanoma: Primary results from the phase 3 IMspire150 trial. 2020 AACR Virtual Annual Meeting. Abstract CT012. Presented April 27, 2020.
- Larkin JMG, et al: 5-year survival outcomes of the CheckMate 067 phase III trial of nivolumab plus i-pilimumab (NIVO1IPI) combination therapy in advanced melanoma, Annals of Oncology 2019; 30: Supplement 5, www.annalsofoncology.org
- Swissmedic, www.swissmedic.ch
- Swiss Drug Compendium, https://compendium.ch
- Dummer R, et al: Encorafenib plus binimetinib versus vemurafenib or encorafenib in pa-tients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19(5): 603-615.
- Direnzo D, et al. : The Immune Checkpoint Inhibitors Unleashed to Fight Cancer. The Rheumatologist, May 17, 2017, www.the-rheumatologist.org
- Hofmann L, et al: Cutaneous, Gastrointestinal, Hepatic, Endocrine, and Renal Side-Effects of anti-PD-1 Therapy. Eur J Cancer 2016; 60: 190-209.
- Liszkay G, et al: Update on Overall Survival in COLUMBUS: A randomized phase III trial of en-corafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in patients with BRAF V600-mutant melanoma. J Clin Oncol 2019; 37 (suppl): Abstr 9512 and poster presentation.
- Gopalakrishnan V, et al: Gut Microbiome Modulates Response to anti-PD-1 Immunotherapy in Melanoma Patients. Science 2018; 359(6371): 97-103.
DERMATOLOGIE PRAXIS 2020; 30(4): 30-31 (published 8/25/20, ahead of print).