Some important and interesting studies in the field of rheumatology in the last year are summarized. As in all fields, technical and analytical capabilities are accelerating exponentially in basic research. As a result, the number of studies published each year in rheumatology is also steadily increasing, and more research and publications are being conducted in all areas of rheumatology than ever before.
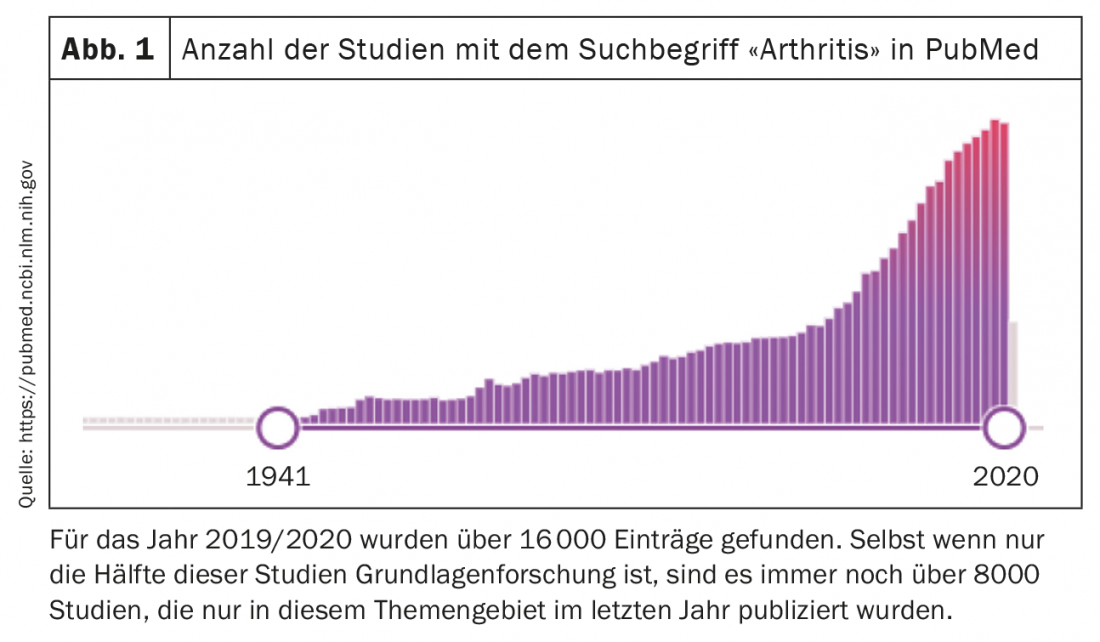
The following article summarizes some important and interesting studies in the field of rheumatology in the last year. It should be noted that the selection of studies is, on the one hand, completely subjective and, on the other hand, naturally incomplete. As in all fields, technical and analytical capabilities are accelerating exponentially in basic research. As a result, the number of studies published each year in rheumatology is also steadily increasing, and more research and publications are being conducted in all areas of rheumatology than ever before. (Fig. 1). However, only time will tell how many of these results will actually have a longer-term impact on our understanding of rheumatologic diseases.
Ultrasound-guided biopsies and single cell analysis
In the analysis of pathogenetic causes, studies of synovial tissue have gained considerable importance in recent years, particularly in the analysis of rheumatoid arthritis (RA), in addition to studies of blood cells and antibodies. On the one hand, the performance of ultrasound-guided synovial biopsies is becoming increasingly common and is routinely performed in many places. On the other hand, advances in the development of technologies for single cell analysis are opening up new possibilities for the differentiated study of changes in individual cell populations in the joint. With these single cell analyses, gene expression, the so-called transcriptome, as well as protein expression and even changes in chromatin can be measured separately in each individual cell. Most studies to date have used measurements of the transcriptome to define cell populations and analyze changes in their composition and gene expression in patients. However, as development progresses and the field becomes more cost-effective, it is likely that there will be more and more studies that simultaneously measure changes in chromatin, transcriptome, and protein expression in individual cells. Single-cell analysis of complex tissue allows characterization of cell types, cell subtypes, and cell phenotypes in a tissue to identify cells that are activated in a disease and thus have a potential key role in pathogenesis (Fig. 2) .
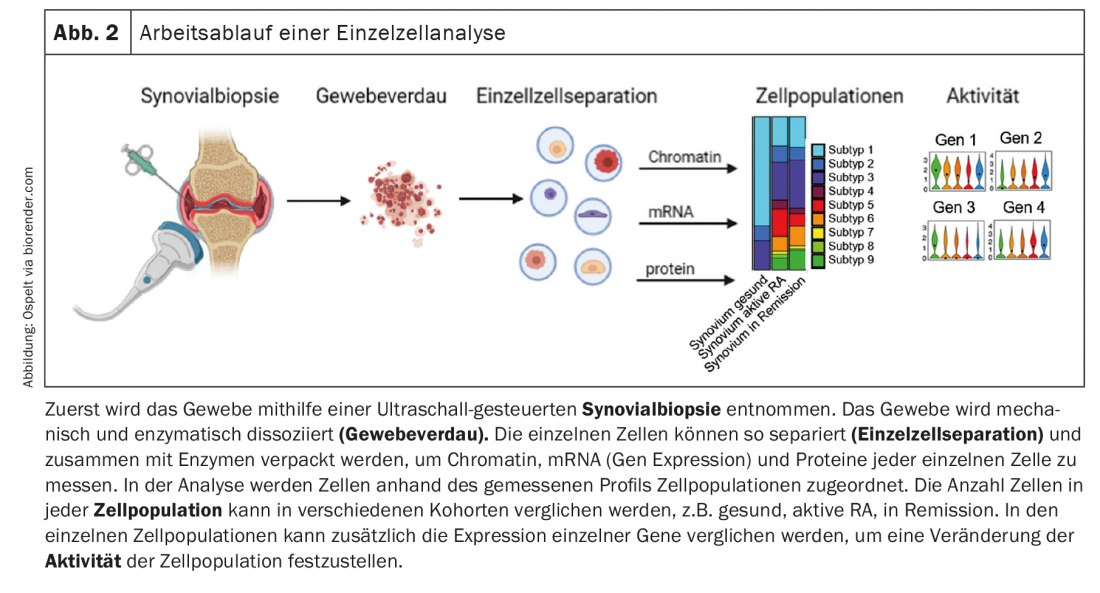
The Accelerated Medicine Partnership (AMP) from the USA is leading the way in this area. This partly industrially, partly governmentally funded consortium has already been able to present quite a few studies in which immune cell types, but also stromal cells in different target tissues and different rheumatic diseases could be characterized. Thus, new immune cell types, with relevance to RA, have already been described in the synovium and different fibroblast subtypes have been defined [1]. These data could be completed in the past year and the formation and function of the different fibroblast populations could be better analyzed.
Interactions between endothelium and fibroblasts in RA synovium.
In their first publication, the authors identified different subtypes of synovial fibroblasts in the tissue [1]. In particular, CD90-negative synovial fibroblasts found in the synovial lining and CD90-positive synovial fibroblasts found in the sublining could be distinguished. The authors now hypothesized that a gradient is established in the synovial tissue that supports the formation of these different fibroblast subtypes [2]. Indeed, ligand-receptor analyses demonstrated that endothelial cells stabilize the CD90-positive fibroblast subtype via activation of the Notch signaling pathway. This activation was stronger in RA tissue than in OA tissue. Using mouse models with Notch3-knock-out mice, respectively pharmacological inhibition of the Notch signaling pathway, the authors demonstrated that Notch inhibition is a potential new target for RA therapy, as arthritis in mice was significantly positively affected by it [2]. Overall, this study fits elegantly with previous studies that have shown that in RA, it is mainly the CD90-positive fibroblasts that expand [1] and that fibroblasts in the lining are more responsible for invasion and fibroblasts in the sublining are more responsible for the inflammatory response [3]. This new study now suggests that activation of the endothelium leads to mobilization of local fibroblast populations in RA, which then keeps inflammation going. In summary, of interest here is, first, the Notch signaling pathway as a potential therapeutic target and, second, the role of endothelial activation in RA, which certainly needs further investigation in the future (Fig. 3).
New macrophage population in synovial tissue.
Our knowledge of macrophage populations in synovial tissue has also been greatly expanded over the past year. Culeman and colleagues studied different populations of marrowrophages in the joint in mice and their behavior during the development of arthritis [4]. Among others, macrophages could be found in the synovial lining (CX3CR1+), which seem to seal off the synovium from the joint interior with their arrangement and cell-cell contacts. When arthritis was induced in mice, this architecture of the lining changed. The macrophages open their cell-cell contacts and now formed palisade. The lining fibroblasts, which in the healthy joint lay beneath this macrophage layer, now virtually squeezed between them and gained more contact with the interior of the joint. These macrophages do not proliferate by division but are likely fed by a pool of proliferating CX3CR1-negative macrophages in the lining that differentiate into this CX3CR1-positive population. Importantly, this morphological change in macrophages was also found in the synovial lining of RA patients, but not in OA patients. In OA, relatively healthy synovium, these macrophages are TREM2-positive, which is a marker for an anti-inflammatory marrowrophage subpopulation. When this macrophage population was depleting in mice, there was a more rapid and severe progression of arthritis. Thus, this specialized macrophage population appears to hold an important barrier function in the joint (Fig. 3). Stabilization of this barrier, for example by imatinib, which stabilizes tight junctions in the blood-brain barrier and also had a positive effect on the occurrence of arthritis in mice in this study, could therefore certainly also be a new therapeutic approach for RA patients.
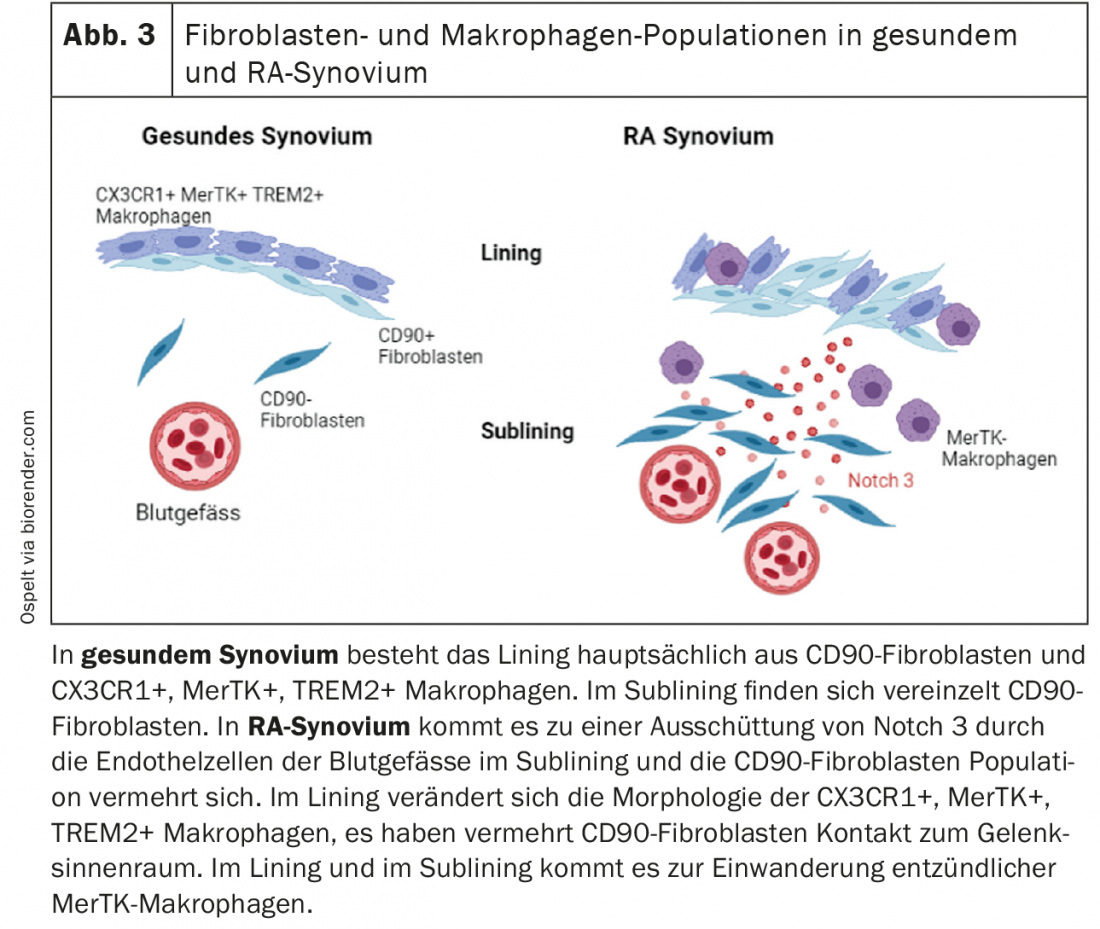
Another study focused intensively on macrophage populations in the joint. Here, synovial tissue from RA patients in clinical remission was compared with patients with active RA [5]. Again, a variety of different macrophage populations were found in synovial tissue. In patients with active RA, so-called MerTK-negative macrophage populations were predominant and showed a pro-inflammatory expression profile. In RA patients in remission, MerTK-positive macrophages in particular, which can additionally express TREM2, were highly enriched. In this regard, these MerTK-positive macrophages found in the synovial lining are likely the TREM2-positive barrier macrophages in the lining previously described in mice and in OA patients in the above study. In co-culture experiments with MerTK-positive macrophages and synovial fibroblasts, it was shown that the fibroblasts produced less pro-inflammatory and joint-destroying factors due to the presence of these macrophages [5]. Conversely, synovial fibroblasts were able to influence the expression of this anti-inflammatory macrophage phenotype. Thus, there seems to be a close interplay between fibroblasts and macrophages in the synovium, which may influence and shape each other at different stages of RA. In the future, it would be interesting to see whether the presence of MerTK-positive macrophages in the synovium is a prognostic biomarker for an increased chance of drug-free remission, and if so, one could start early to reduce therapy in these patients without risking a disease relapse.
Prediction of disease relapses in RA patients
The prognosis of disease relapses in RA patients was addressed in a study published in the New England Journal of Medicine [6]. Here, a drop of blood was drawn weekly from the fingertip of 4 patients over a period of 1 to 4 years, and monthly measurements of disease activity were performed. The blood from these blood drops was sequenced to find a pattern of gene expression before and during a disease episode, respectively. In fact, two gene clusters could be identified that were always measurable in the blood before a relapse. Based on the genes that were regulated in these clusters, it was possible to guess that one probably came from immune cells. The other, however, surprisingly resembled more a profile known from synovial fibroblasts. It was possible to isolate these “pre-inflammatory mesenchymal cells” (PRIME) cells from the blood in additional RA patients and to show that these PRIME cells are indeed the cell population that produces the gene signature we are looking for. Thus, these data could mean that mesenchymal cells, e.g., synovial fibroblasts from the joints, leak into the blood before a relapse, and this process is a harbinger of the relapse or triggers the relapse. However, the origin and role of these cells in RA remains completely unclear to date and will certainly be addressed in further future studies.
New insights into the pathogenesis of Systemic Lupus Erythematosus (SLE).
It has been known for some time that a special subpopulation of granulocytes called low-density granulocytes (LDG) is present in the blood of patients with SLE, and it has been suggested that these play a role in the development of the interferon signature in SLE. In a recent analysis, it was shown that monocytes on the one hand and LDGs on the other hand are responsible for the high production of interferon response genes in SLE patients [7]. Furthermore, using single cell analyses, the study was able to show that this LDG subpopulation can also be subdivided again and that these different phenotypes correlate with different clinical parameters. Two main subtypes of LDG were distinguished, those that are CD10-positive and those that are CD10-negative. Since CD10 is a maturation marker for neutrophil granulocytes, CD10-negative LDG is likely an immature precursor. These immature neutrophil granulocytes were unable to make nuclear extracellular traps (NET) in vitro, showed less chemotactic and phagocytotic activity than CD10-positive granulocytes, but in contrast produced more myeloperoxidase, indicating a higher capacity for degranulation. Accordingly, mainly CD10-positive mature LDG correlated with clinical parameters, such as organ damage and glomerular filtration rate. This suggests that the very functions that mature CD10-positive LDGs perform, such as NET formation, chemotaxis, and phagocytosis, play a role in the pathogenesis of these symptoms.
A new approach to clarify how this NET formation might be triggered in SLE was presented by a group of researchers from Bethesda, USA [8]. This group was able to show that voltage-dependent anion channels (VDAC) oligomerize and, stabilized by free mitochondrial DNA, form a channel in the mitochondrial membrane from which mitochondrial DNA can exit into the cytoplasm. This mitochondrial DNA triggered an interferon response in the cytoplasm on the one hand and led to the formation of NET in the LDGs already mentioned above on the other. In blood cells from SLE patients, these mitochondrial channels were found with increased abundance and in a lupus mouse model, blocking this channel formation in mitochondria resulted in less mitochondrial DNA in the cytoplasm, less interferon production, less NET formation, and improved clinical parameters. This treatment also reduced the formation of NET in granulocytes from SLE patients. Thus, blocking this channel protein could be a new promising approach in the therapy of SLE.
B-cell analysis provides insights into the development of immune-mediated diseases
New technologies also helped to analyze in detail B cells and their receptors in different immune-mediated diseases [9]. Here, B cells from patients with ANCA-associated vasculitis (AAV), SLE, Mb. Crohn’s, Mb. Behçet’s disease, eosinophilic granulomatosis with polyangiitis (EGPA, formerly Churg-Strauss syndrome), and IgA vasculitis. The study looked at the isotypes, the genes of the IGHV locus, which is the B cell receptor heavy chain, and the clonality of the B cell repertoire and discovered some interesting things. IgA antibodies were overrepresented in all diseases studied except AAV and EGPA. Since IgA secretion is mainly stimulated via the immune system of the mucosa, e.g. in the intestine, it was expected that in patients with Mb. Crohn’s, the IgA content is high. However, it was surprising that very high IgA levels were also measured in patients with SLE. This could also indicate stimulation of the mucosa, e.g. in the intestine, in SLE. Analysis of genes encoding the immunoglobulin heavy chain variable region also revealed interesting differences. The expression of IGHV6 and IGHV4 genes was particularly high in SLE, EGPA, and Mb. Crohn’s increased. These genes have previously been associated with autoreactivity. Unusually, the high expression of IGHV1 genes in B cells from patients with Mb. Behçet, as the overproduction of these genes are mainly associated with infections. This supports the hypothesis that infection may precede the onset of Mb. Behçet could be underlying. Examination of the clonality of B cells in the different diseases showed that in patients with SLE and Mb. Crohn’s, the expansion of the clones as well as the diversity of the B-cell clones was increased. Although this was so expected, it shows conversely that in the other diseases, despite strong activation of the immune system, B cell clonality is normal. Furthermore, this study also examined changes in the B-cell repertoire after therapy, showing that different immunosuppressive treatments have different effects on the B-cell repertoire. Treatment with mycophenolate mofetil resulted in an increased proportion of IgM and IgD-producing B cells and thus a decrease in the number of B cells that underwent an isotype switch. In contrast, after rituximab therapy, the number of circulating B cells decreased sharply, but the persistent B cells had largely changed isotype and expanded clonally. In AAV, IgA-producing B cells were predominantly found after rituximab; in SLE, IgG1 or IgG2.
Summary
In summary, the latest analytical methods on a single cell basis in particular have led to new insights into the function of certain cell subtypes in both healthy and diseased tissue. We can thus differentiate the pathological processes in the various rheumatic diseases more and more precisely, which will hopefully also lead to new therapies for the individual diseases in the future. In rheumatology in particular, the step from basic research to new treatment methods has already been successfully taken many times.
Take-Home Messages
- Ultrasound-guided synovial biopsies and single-cell analyses have become an important component of translational research.
- Pharmacological Notch inhibition and stabilization of macrophage tight junctions in synovial lining are novel therapeutic targets for RA.
- NET formation and production of interferon genes in SLE by a specific subtype of neutrophil granulocytes correlates with clinical symptoms.
- Inhibition of mitochondrial membrane channel formation is a novel therapeutic target in SLE.
Literature:
- Zhang F, et al: Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol 2019; 20: 928-942.
- Wei K, et al: Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 2020; 582: 259-264.
- Croft AP, et al: Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019; 570: 246-251.
- Culemann S, et al: Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 2019; 572: 670-675.
- Alivernini S, et al: Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat Med 2020; 26: 1295-1306.
- Orange DE, et al: Identification of Three Rheumatoid Arthritis Disease Subtypes by Machine Learning Integration of Synovial Histologic Features and RNA Sequencing Data. Arthritis Rheumatol 2018; 70: 690-701.
- Mistry P, et al: Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci USA 2019; 116: 25222-25228.
- Kim J, et al: VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 2019; 366: 1531-1536.
- Bashford-Rogers RJM, et al: Analysis of the B cell receptor repertoire in six immune-mediated diseases. Nature 2019; 574: 122-126.
InFo PAIN & GERIATry 2021; 3(1): 6-9.