Atezolizumab enables long-term survival for some pretreated lung cancer patients. Alectinib also helps patients live a more tolerable daily life by mitigating disease symptoms over the longer term. These are the findings of two ELCC studies.
The phase II trial, called POPLAR, randomized 287 patients from a total of 13 countries. They all suffered from an advanced form of non-small cell lung cancer (NSCLC) and had already received treatments for it. One part underwent immunotherapy with the anti-PD-L1 antibody atezolizumab, the other chemotherapy with docetaxel.
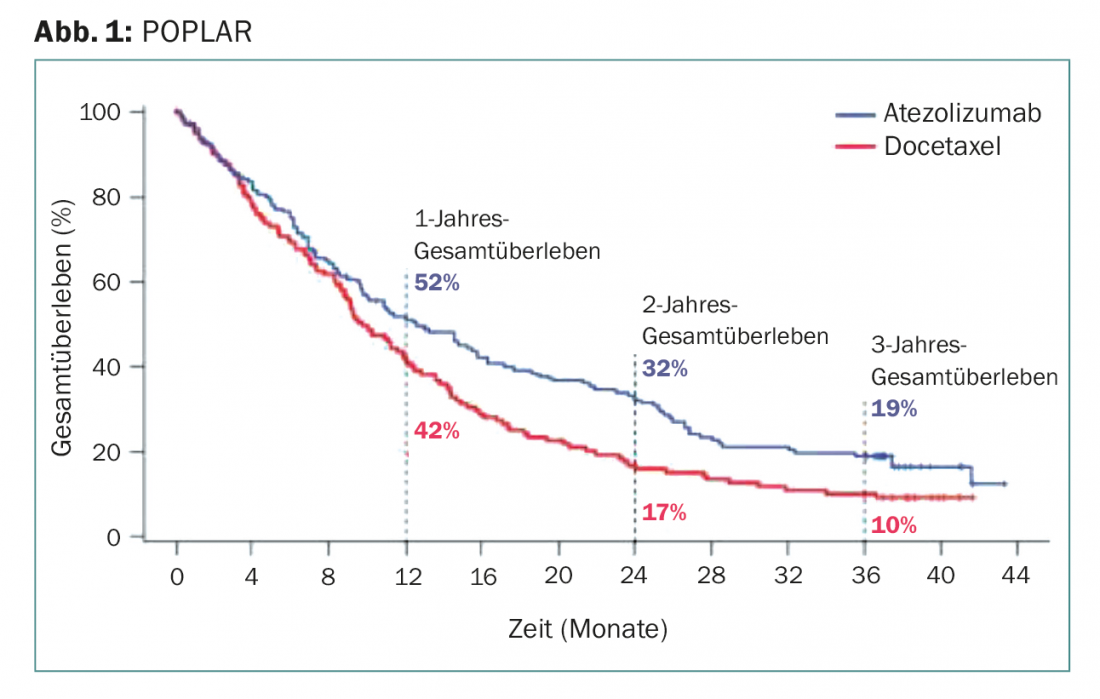
At two and three years – the longest follow-up to date of PD-L1 immunotherapy in this setting – atezolizumab was significantly superior to docetaxel (Fig. 1):
- Approximately one-third from the former group were still alive after two years. In the docetaxel group, the survival rate was a good half lower (16.6%)
- Even after three years, immunotherapy still increased survival twofold, from 10% with docetaxel, to 18.7% with atezolizumab
- The benefit was shown to be independent of prospectively evaluated PD-L1 expression and histology (squamous cell carcinoma or not). Even patients with PD-L1 expression in less than 1% of tumor and immune cells still benefited.
In addition, there is a threefold increase in response duration (with the same high overall response rate of 15%) and fewer adverse events with immunotherapy. The test substance was well tolerated (enough) to be administered for several years. So, with immunotherapy, some patients are now considered “long-term lung cancer survivors,” are back at work, and have a more or less good quality of life.
The efficacy data from POPLAR were confirmed in the phase III trial called OAK [1].
What conclusions can be drawn from POPLAR?
While atezolizumab appears to be an option in all patients with advanced NSCLC based on the subgroup analyses from POPLAR. However, this also makes it difficult to predict exactly which patients will benefit from the active substance in the long term. It is not known exactly who this fifth of patients is who are still alive after three years and how they are characterized. Thus, the definition of predictive biomarkers remains a challenge.
At the same time, the number of approximately one-fifth of 3-year survivors represents an exceedingly good outcome in this collective. Atezolizumab is thus elevated to the ranks of agents with the highest survival rates ever in previously treated lung cancer patients. Before immunotherapy, there were virtually no long-term survivors with NSCLC. Now, in light of new study data, this is becoming possible for the first time. The POPLAR results are flanked and supported by the comparable 3- and 5-year survival rates from studies with the anti-PD1 antibodies pembrolizumab and nivolumab [2]. Under the latter, about 15% of patients were still alive after five years (phase I), which is usually already considered “cured” in cancer. POPLAR has critical relevance as a large randomized trial that now confirms the long-term survival benefit for the PD-L1 approach.
If one looks at the entire study situation, there is now a strong argument in favor of every NSCLC patient with advanced forms receiving immunotherapy. Finally, the chance of 5-year survival appears to be approximately 1:6. Unfortunately, as is so often the case in recent times, financial questions remain unanswered. How could our health care system stem a broader prescription of immunotherapy? Probably only if one could identify patients who would certainly not benefit from such a strategy. Now, however, PD-L1 expression in POPLAR has again proven to be an inadequate biomarker. If individuals with extremely low expression levels still benefit survival-wise, it alone cannot serve to exclude them. Currently, biomarker research is in full swing. In the future, it is likely that a “bundle” of biomarkers will be increasingly used, possibly including tumor mutational burden. However, regarding combination of biomarkers, there is a need for studies conducted in similar collectives as those of the aforementioned studies on atezolizumab, pembrolizumab, and nivolumab. So the long-term survivors there would first need to be defined precisely (demographically, smoking history, tumor mutation burden, immune response, expression level, etc.) and in a next step look at which biomarkers (together) are most predictive.
Atezolizumab (Tecentriq®) is currently approved in Switzerland for the treatment of patients with locally advanced or metastatic NSCLC, following prior chemotherapy. Treatment requires approval of costs by the health insurer after prior consultation with the medical examiner.
News about ALEX
The ALEX trial [3], published last August, evaluated the selective ALK and RET kinase inhibitor alectinib in phase III. This is considered a “next generation” tyrosine kinase inhibitor. In a sample of 303 NSCLC patients with previously untreated advanced disease and oncogenic driver mutation (ALK), the investigational agent demonstrated superior efficacy and safety/tolerability compared with the previous standard tyrosine kinase inhibitor crizotinib. Overall, approximately 4% of all NSCLC patients are ALK-positive and thus more susceptible to CNS metastases. Among other findings, the head-to-head study significantly reduced the risk of progression or death by more than 50%, and CNS progression was also significantly less frequent (12% vs. 45%, HR 0.16, p<0.001).
At the ELCC in Geneva, the focus was now on outcomes from ALEX that could be self-assessed by patients, i.e., disease burden, symptom tolerability, and health-related quality of life. For this purpose, the validated questionnaire QLQ-C30 of the EORTC was used with the modular appendix QLQ-LC13, which was specifically developed for lung cancer patients. Corresponding symptoms of illness can thus be surveyed in a 13-part questionnaire. Baseline characteristics of assessable patients (approximately two-thirds each of the alectinib and crizotinib groups) were comparable. Patients completed questionnaires at baseline and monthly thereafter, and also within one month of withdrawal from the study (although therapies were rarely discontinued due to symptom worsening) and after disease progression.
Both agents led to a significant improvement in disease symptoms. Considering the symptom complex consisting of cough, dyspnea, and chest pain (which is commonly a high burden for advanced lung cancer patients), it took approximately the same median time for symptom worsening to occur under the two agents. However, on average, patients found that their baseline symptoms were improved over a longer time when they received alectinib instead of crizotinib (consistent with the beneficial PFS data with alectinib). For example, cough improved clinically significantly over an average of 96 weeks (vs. 84 weeks in the comparison group), chest pain over 96 vs. 80 weeks, and pain elsewhere or fatigue over 96 vs. 68 weeks, respectively.
Quality of life was clinically significantly improved in both groups, but over a longer period with alectinib, 88 weeks versus 68 weeks. Significant worsening in terms of treatment-associated symptoms such as nausea, vomitus, diarrhea, loss of appetite, dysphagia, peripheral neuropathy, etc. occurred in fewer alectinib patients than crizotinib patients. Overall, patient self-documented data were consistent with previously published safety data, confirming improved tolerability with alectinib. They are also consistent with efficacy data: both arms had shown comparable response rates, albeit with longer response duration under alectinib. Similarly, quality of life and lung cancer symptoms were improved in both arms, but the improvements lasted longer with alectinib.
Patients with ALK-positive NSCLC are at particularly high risk of developing multiple brain metastases, as mentioned above. Radiation options are limited for this distressing symptomatology, and cognitive functions may also be impaired as a result. Alectinib is associated with a high level of protection against brain metastases; it reduces the likelihood that existing cerebral metastasis will progress. The mechanisms behind this are currently being researched [4]. When only those patients with CNS metastases at baseline were considered in the subanalysis of patient data presented at ELCC, alectinib was also superior: The benefits in terms of progression resp. the strong CNS activity with the active substance were consequently reflected in the daily life of the patients. On the one hand, quality of life decreased less frequently, and on the other hand, about half as many affected persons reported cognitive deterioration (17.9% vs. 34.6% at week 32).
Thus, there is much to be said for a new standard-of-care in the first-line setting of ALK-positive advanced-stage NSCLC patients. For the latter group, Alecensa® is currently also approved in Switzerland, but according to the Limitatio only after progression under crizotinib or in case of crizotinib intolerance and after cost approval by the health insurer after prior consultation with the medical officer.
The fact that patient data are collected and evaluated systematically at all in a large phase III trial is of central importance. Quality of life and burden of disease may even be more important than prolonged survival per se (and “at any cost”), especially in the palliative situation. Active substances that not only extend the remaining life span, but also make it more worth living, are therefore highly welcome.
Source: ELCC 2018 (European Lung Cancer Congress), April 11-14, 2018, Geneva.
Literature:
- Rittmeyer A, et al: Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017 Jan 21; 389(10066): 255-265.
- Vokes EE, et al: Nivolumab versus docetaxel in previously treated advanced non-small cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018 Feb 2. DOI: 10.1093/annonc/mdy041 [Epub ahead of print].
- Peters S, et al: Alectinib versus crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017 Aug 31; 377(9): 829-838.
- Kodama T, et al: Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014 Nov; 74(5): 1023-1028.
InFo ONCOLOGY & HEMATOLOGY 2018; 6(3) – published 8.6.18 (ahead of print).











