The term “benign prostatic syndrome” (BPS) describes micturition symptoms associated with the prostate. The term “benign prostatic hyperplasia” (BPH), formerly frequently used to describe micturition symptoms, describes only a histological picture and has been replaced by the term LUTS (lower urinary tract symptoms). Basic diagnostics include history, physical examination including palpation of the prostate, questionnaire (IPSS), sonography and urine status. Advanced diagnostics include cystoscopy and cystomanometry. The choice of a suitable drug or surgical therapy depends, among other things, on the symptoms and the size of the prostate.
Lower urinary tract symptoms (“LUTS”) are divided into obstructive and irritative micturition symptoms. These are bladder emptying or bladder storage symptoms (Table 1) [1].
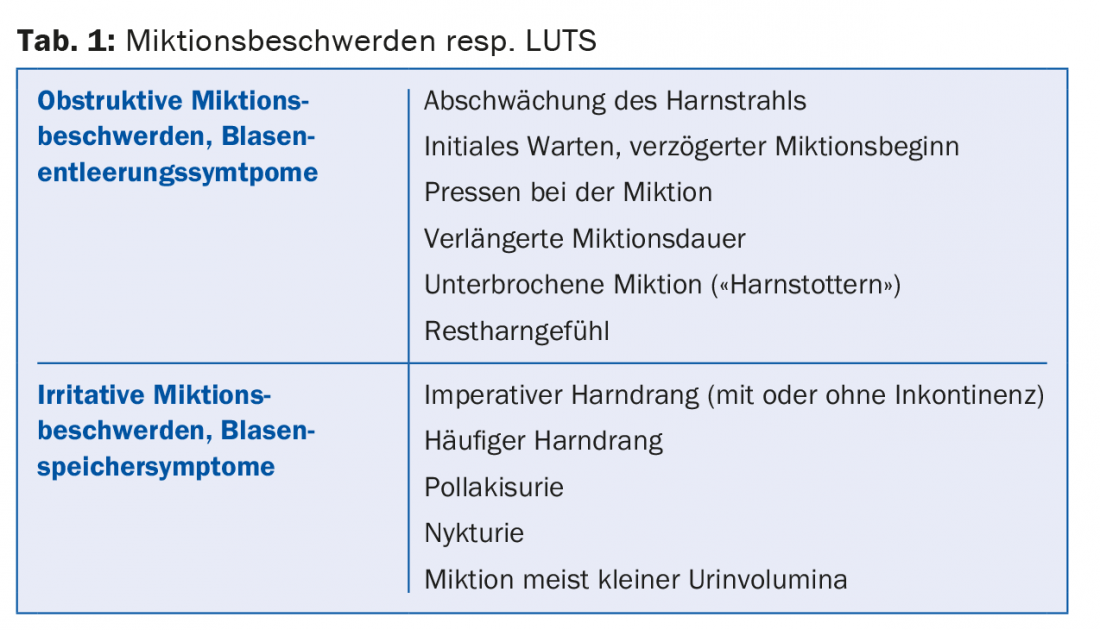
In clinical practice, patients not infrequently present with a combination of these complaints. Infravesical obstruction due to prostatic enlargement is a common cause of micturition symptoms in men, but numerous differential diagnoses must be considered (Table 2). The use of the descriptive term LUTS takes this fact into account. The term BPH (benign prostatic hyperplasia), which used to be frequently used in connection with micturition problems in men, merely describes a histological picture and should no longer be used to describe micturition problems.
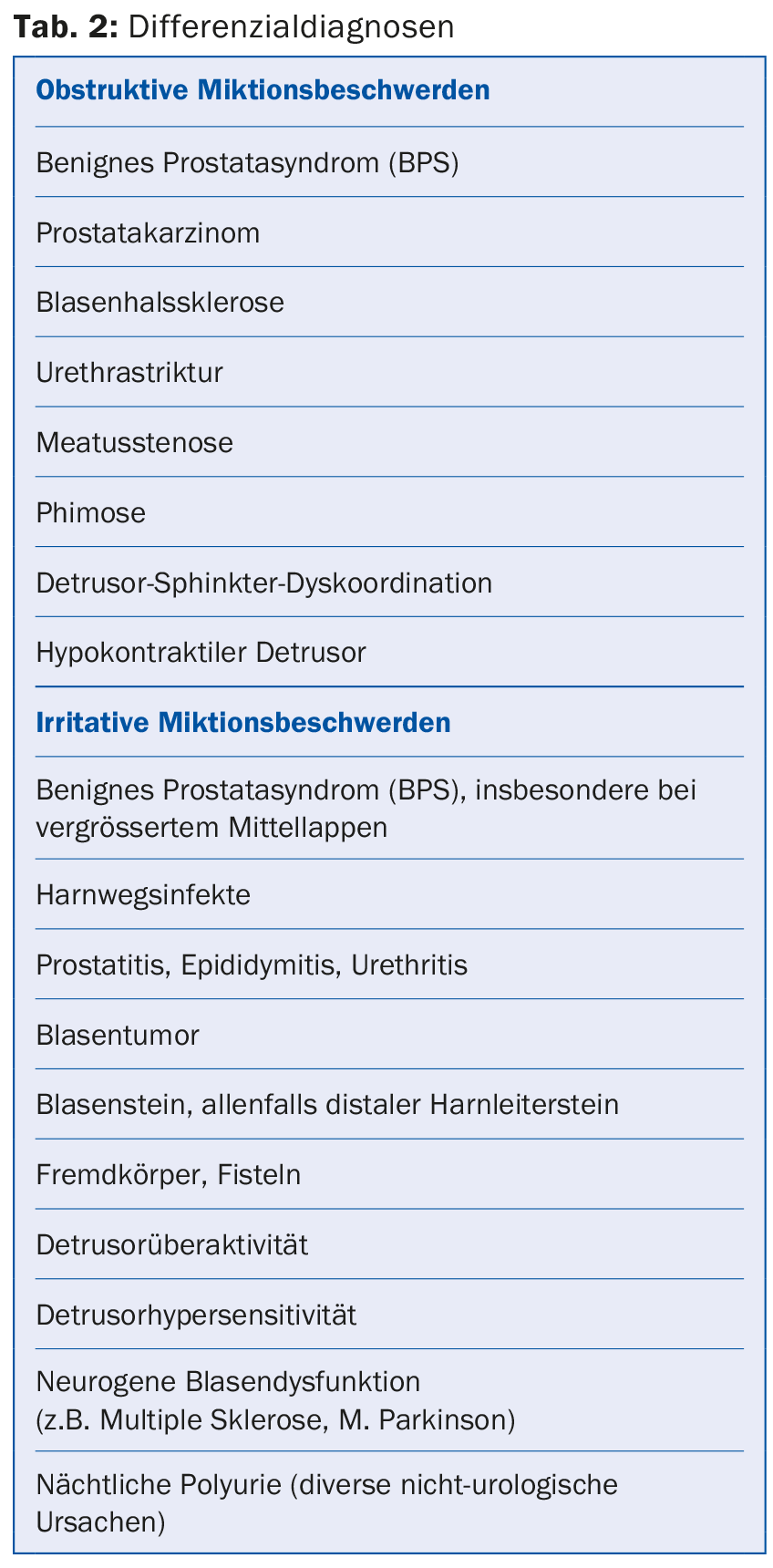
If prostate enlargement is the cause of micturition problems and there is no suspicion of prostate cancer, we can speak of benign prostate syndrome (BPS). This term considers the pathophysiologic relationships of prostate enlargement and any resulting bladder outlet obstruction. The aim of this article is to take a closer look at obstructive micturition symptoms in this context and to provide an overview of diagnostics and therapy.
Basic diagnostics
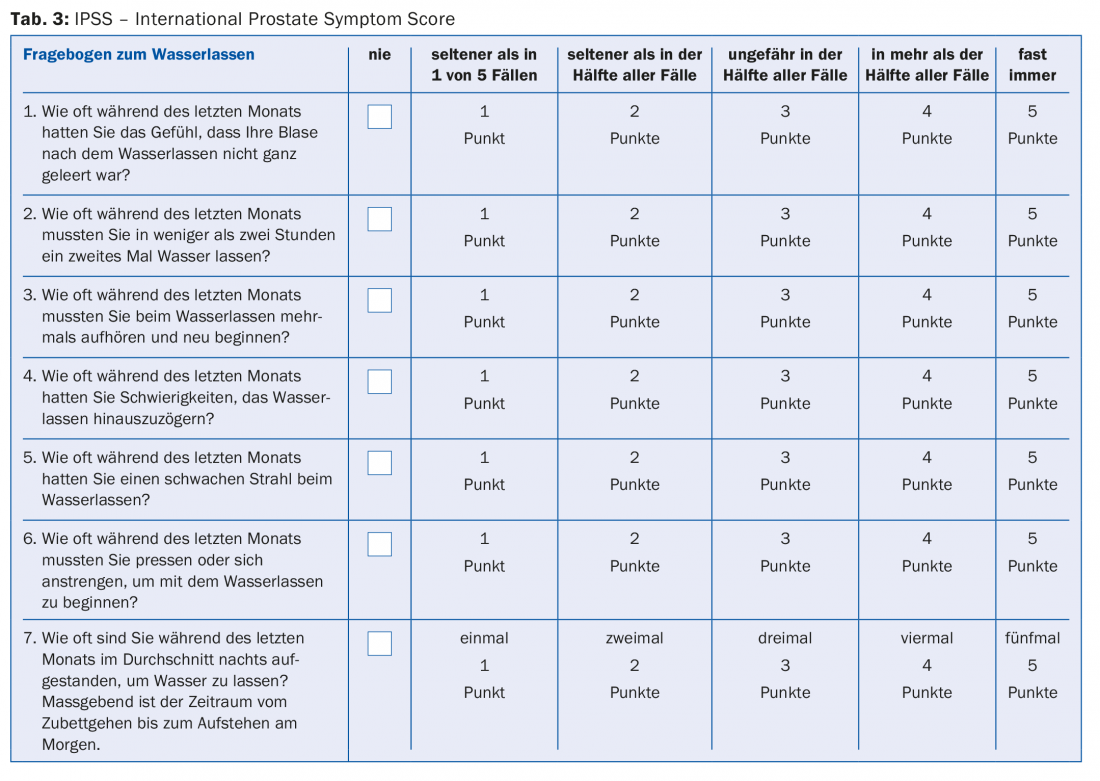
The basis of the diagnosis is the medical history. This includes questions about the type and onset of symptoms, medications, concomitant diseases, and any surgeries. Helpful and therefore important part of the anamnesis should be the use of a standardized and validated questionnaire. The IPSS questionnaire (“International Prostate Symptom Score”) helps to objectify the symptoms and also provides an opportunity to evaluate any therapeutic success during the course of treatment (tab. 3) . A micturition diary should also be kept, especially in the case of irritative micturition symptoms. This always includes the patient’s drinking habits and can help to distinguish nocturia from nocturnal polyuria, among other things.
The physical examination includes examination of the external genitalia (exclusion of phimosis, meatus stenosis, epididymitis, etc.) and a digital-rectal examination to assess the size, consistency, pressure dolence and delineation of the prostate. Urinalysis (strip test or ideally urine sediment) detects a possible urinary tract infection or microhematuria. Determination of prostate specific antigen (PSA) is recommended when the result would either influence the LUTS treatment decision or the diagnosis of prostate cancer would have a therapeutic consequence in the patient.
Uroflowmetry is a further diagnostic method usually only available to urologists. This allows the urinary stream to be assessed and can often already provide clues to possible differential diagnoses. Transabdominal sonography is used to determine the amount of residual urine after micturition and also to assess the upper urinary tract to detect any urinary transport disorder. Prostate size may have therapeutic consequences and should therefore be routinely determined. Transrectal sonography is particularly suitable for this purpose, although an orientational assessment is also possible by means of transabdominal sonography. Knowing the size of the prostate helps in selecting an appropriate medication or surgical method.
Further diagnostics
Uroflowmetry helps the urologist to make a tentative diagnosis in many cases. For example, a so-called “plateau flow” is compatible with urethral stricture, an arcuate attenuated curve with a prostatogenic voiding disorder. Urethrocystoscopy is used to exclude pathologies in the area of the bladder as well as urethra. This examination should be performed especially in the presence of microhematuria or macrohematuria and also in the presence of unexplained or refractory irritative micturition symptoms. For example, it is possible to diagnose urethral strictures or bladder neck sclerosis, as well as to evaluate the prostatic urethra. The diagnosis of a bladder tumor or bladder stones is also easily made by cystoscopy. Although infravesical obstruction cannot be proven by urethrocystoscopy, certain changes can be detected as indirect evidence of it, e.g. trabecularization as well as diverticula of the urinary bladder wall.
Only urodynamic examination (cystomanometry) allows detection of obstruction by assessing the micturition profile (obstructive vs. non-obstructive). In general, this examination can be used to assess the function of the detrusor, the interaction of the detrusor and sphincter, and the distensibility, irritability and capacity of the bladder. A urodynamic evaluation can be considered for micturition problems that remain unclear despite basic diagnostics or if treatment is not successful. It may also be useful to prove an obstructive micturition profile before indicating surgery in cases of concomitant neurologic disease.
Watchful waiting
A wait-and-see approach may be considered in men with mild symptoms (IPSS score 0-7) and low distress [1,2]. Regular monitoring and possible behavioral adjustments are important, e.g., in the case of nocturia, a reduction in the amount drunk in the evening, especially of diuretic beverages.
Drug therapy
Drug therapy should be evaluated especially in the case of IPSS score >7 and lack of absolute indication for surgery. The goal of treatment is on the one hand symptom relief, and on the other hand, if possible, inhibition of progression.
α1-Receptor antagonists (e.g., tamsulosin, alfuzosin, terazosin) are a well-established symptomatic therapeutic option for BPH-associated voiding symptoms. Both symptom score (IPSS) and urinary stream strength are improved [3]. Alpha blockers work regardless of prostate size and usually work quickly. They have no effect on the natural course of the disease (progression), prostate size or PSA level. Possible side effects due to vasodilation include dizziness, fatigue, and hypotension, and retrograde ejaculation may also occur. Before any cataract surgery, the ophthalmologist should be informed about the intake, as intraoperative “floppy iris syndrome” (IFIS) may occur [4].
5-α-Reductase inhibitors (e.g., finasteride, dutasteride) decrease the intraprostatic concentration of dihydrotestosterone, resulting in a significant decrease in prostatic volume within 6-12 months and thus consecutive reduction in bladder outlet obstruction. 5-α-Reductase inhibitors should be considered as long-term therapy, especially for prostate volumes greater than 40 ml. Unlike alpha blockers, these drugs do not affect smooth muscle tone, and the effect is delayed. Both symptom score (IPSS) and urinary stream strength are improved [3]. Unlike alpha blockers, 5-α-reductase inhibitors have a positive impact on the natural history of the disease. This was shown in studies by a reduced risk of acute urinary retention and the need for surgery [3]. Possible side effects include loss of libido, erectile dysfunction, and rarely gynecomastia. It is important to note that 5-α-reductase inhibitors affect PSA levels and serum PSA levels can decrease by more than half with therapy. A PSA increase during ongoing therapy with 5-α-reductase inhibitors should always be clarified urologically and a possible prostate carcinoma should be excluded.
Combination therapies: The combination of alpha blocker and 5-α-reductase inhibitor utilizes the different mechanisms of action, resulting in a synergistic therapeutic effect in terms of symptom relief and progression inhibition. Studies have shown that this combination is particularly useful in patients at increased risk of progression [3]. Especially from a certain prostate volume (>40 ml) this therapy option is reasonable, also patient age as well as PSA value can be used to estimate the risk of progression [2,5]. Dutasteride and tamsulosin are available in Switzerland as a combination preparation in a capsule. The alpha blocker can be discontinued after six months on a trial basis. Also possible is the combination of alpha blocker and anticholinergic [6], especially in mild obstructive micturition symptoms with urge symptoms in the foreground.
Anticholinergics (e.g., trospium chloride, solifenacin succinate, tolterodine, etc.) have no role as monotherapy for purely obstructive micturition symptoms, but may be combined with an alpha blocker if irritative symptoms are also present. Sonographic residual urine monitoring is recommended both before starting and during ongoing therapy.
β3-Receptor Agonists: The mechanism of action of the relatively new drug mirabegron allows treatment of bladder storage symptoms; therefore, it is not discussed in detail here.
PDE-5 inhibitors (e.g., tadalafil) are a relatively new therapeutic option for the treatment of LUTS and, to date, have been known primarily for the treatment of erectile dysfunction (ED). They are newly mentioned in the current EAU guidelines. In Switzerland, only tadalafil is currently approved at a dosage of 5 mg daily. However, long-term data on the influence on the natural course of the disease, prostate volume and PSA level are lacking, as are data on the exact mechanism of action. The effect appears to be independent of an existing ED. The known side effects must be taken into account.
Phytotherapeutics are a very heterogeneous group of preparations and active substances. Based on the current data situation, no uniform recommendation can be made.
Surgical therapy
The indication for surgery is not infrequently a matter of judgment. The possibility of surgery must be discussed individually with the patient. However, there are absolute indications such as recurrent urinary tract infections, recurrent urinary retention, bladder stones, upper urinary tract dilatation with or without renal insufficiency, and recurrent prostatogenic macrohematuria.
An important criterion in the selection of the optimal surgical procedure is the prostate volume. The reference technique for glands of 30-80 ml is transurethral prostatic resection (TUR-P) with its different modifications (monopolar or bipolar) [2,7]. Postoperatively, there is often retrograde ejaculation, which is not a complication but a normal consequence of surgery. Every patient should be informed about this preoperatively. Laser techniques (e.g., holmium, thulium, greenlight, etc.) allow surgery even with continued oral anticoagulation or dual antiplatelet therapy with ASA and clopidogrel. For large glands, according to EAU guidelines >80 ml, open prostate adenoma enucleation via a lower abdominal incision can be considered as a very effective procedure. Also possible as a transurethral procedure for large glands is, for example, holmium laser enucleation of the prostate (HoLEP). While this procedure has lower morbidity, it also has a significant learning curve and is not widely available.
Literature:
- Sarma AV, Wei JT: Clinial Practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N Engl J Med 2012; 367(3): 248-257.
- Oelke M, et al: EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013; 64(1): 118-140.
- McConnell JD, et al: The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25): 2387-2398.
- Michel MC, et al: What does “intraoperative floppy iris” syndrome mean for urologists? Der Urologe 2006; 45(12): 1547-1548.
- Crawford ED, et al: Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. J Urol 2006; 175(4): 1422-1426.
- Kaplan SA, et al: Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296(19): 2319-2328.
- Engeler DS, et al: Bipolar versus monopolar TURP: a prospective controlled study at two urology centers. Prostate Cancer Prostatic Dis 2010; 13(3): 285-291.
HAUSARZT PRAXIS 2015; 10(5): 26-30