Hodgkin’s disease belongs to the group of malignant lymphomas and is manifested by painless swelling of the lymph nodes, which are very often initially found on the neck or behind the breastbone. Even in advanced stages, treatment can still be effective.
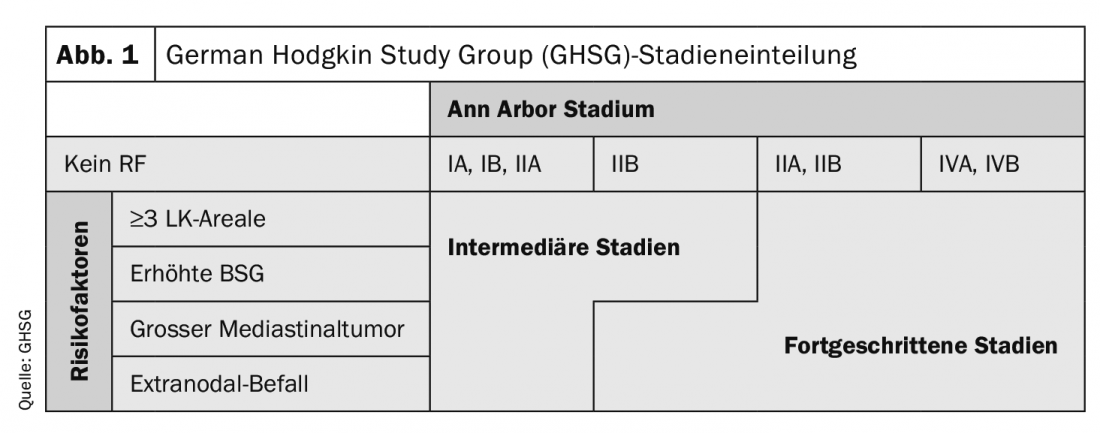
Patients with Hodgkin’s lymphoma (HL) are additionally classified into early, intermediate, and advanced stages according to their risk factors, in addition to the Ann Arbor stages (Fig. 1). The type and intensity of therapy are based accordingly on the stage present . PET can now also be used for initial staging, which has a very clear advantage over contrast-enhanced computed tomography in terms of sensitivity of detection of bone marrow involvement.
Early stage CMT strategy
Standard therapy of HL in early stages is the combined modality treatment (CMT) strategy. In this case, chemotherapy is administered with 2-4 cycles and the radiation dose ranges from 20 Gy to 30 Gy depending on the exact stage. Currently, involved-site therapy (IS-RT) is recommended, although data from prospective studies are not yet available. The fields are clearly more circumscribed here. Usually (>90%), HL manifests supradiaphragmally and therefore the radiation fields often include the heart, great vessels, thyroid, or lung and breast tissue. “Because of the potential long-term side effects of radiation therapy, including cardiovascular (coronary artery disease and myocardial infarction, heart failure) and oncologic (breast cancer, lung cancer), this modality of therapy is being questioned, particularly by hematologists,” Borchmann opined. On the other hand, consolidative radiotherapy, for example, allows reduction of chemotherapy to only 2 cycles of ABVD in early stages, thus requiring minimum intensity from both modalities for optimal lymphoma control. Long-term data published in 2017 from the early-stage GHSG trials with approximately 10 years of follow-up have not shown a relevant increased incidence of secondary neoplasia, so with the available evidence there is no reason to deviate from the combined modality concept.
Another option is to improve the ABVD regimen by adding new compounds. “Here, brentuximab vedotin has been the most promising substance in recent years with a good benefit-risk ratio as a monotherapeutic,” the expert reported. Since bleomycin is carried in the ABVD regimen more for historical reasons and has low efficacy with relevant toxicity, this agent was replaced by brentuximab vedotin (BV+AVD). The study included 170 patients with a mean age of 29 years. The primary endpoint was the rate of PET-2-negative patients, which is 75% with ABVD alone and should be 85% with the new regimen, excluding a PET-2-negative rate of <75%. After 2 cycles of treatment, 93/113 patients (82.3%, 95%CI 75.3-88.0) and 43/57 (75.4%, 95%CI 64.3-84.5) achieved negative PET, based on central review in the experimental and standard arms, respectively. With the lower limit of the 95% confidence interval greater than 75% (actually it was 75.4%) in the experimental arm, the main objective can be considered fulfilled. However, grade 3 or 4 events were documented during the first two cycles in 74% of patients in the BV-AVD arm and in 56% in the ABVD arm. The authors conclude that the PET-2 negative rate with BV-AVD is within the target range, but this is associated with significantly increased toxicity with BV-AVD treatment compared with ABVD even during the first two cycles of treatment.
PD1 antibodies also suitable in the first line?
The PD1 antibody nivolumab is approved for relapsed or refractory classical Hodgkin’s lymphoma (cHL) and has a high overall response rate (ORR) and favorable safety profile. To evaluate the efficacy of nivolumab in combination with doxorubicin, vinblastine, and dacarbazine (AVD) as first-line treatment for intermediate classical HL (cHL), a prospective, randomized phase II study was conducted. Treatment included therapy-naive cHL patients in intermediate stages. Patients in Arm A received 240 mg nivolumab and AVD at standard doses on days 1 and 15 of each 28-day cycle for a total of four cycles (4× NivoAVD). In arm B, the same treatment was given sequentially, starting with 4× nivolumab 2 weeks apart, followed by 2× NivoAVD and 2× AVD. Both groups received 30 Gy of involved-site radiotherapy (IS-RT). The primary endpoint was PET/CT-based CRRate after completion of protocol therapy including IS-RT.
The results showed that adverse events (AEs) were recorded in 98% of patients. Grade 3 or 4 adverse events occurred in 73% and 78% of cases, respectively, and severe AEs were observed in 38% and 28% of patients, respectively. After completion of systemic therapy, ORR was 100% (54/54) and 98% (50/51) with CR rates of 81% and 86%, respectively. “The study shows an unexpectedly high CR rate after nivolumab monotherapy in first-line intermediate-stage cHL,” Borchmann opined. “The high and very rapidly achieved CR rate after combination therapy of nivolumab and AVD suggests synergism of the different therapeutic principles.” The study authors believe that in intermediate stages, concurrent or sequential therapy with nivolumab and AVD is possible with acceptable toxicity.
Effectively treat advanced stages
Disseminated lymph node involvement or organ involvement, i.e., stage III or IV according to Ann-Arbor, are considered advanced stages of Hodgkin lymphoma. The international standard is six to eight cycles of ABVD followed by consolidative radiotherapy on initial bulk or residual tumors greater than 1.5 cm in diameter, although again without evidence from prospective studies, PET-guided radiotherapy is often used in clinical practice. “However, since the GHSG-HD15 publication, six cycles of BEACOPPescalated followed by radiotherapy have been standard in the GHSG for patients with PET-positive residual tumors greater than 2.5 cm,” the expert indicated. This approach achieves an overall survival of 95% at five years, which has not been previously described in any other study worldwide. But there are also criticisms: the BEACOPPescalated regimen leads to overtreatment of all those patients who could also have been cured with a less intensive therapy such as ABVD. This is about 2/3 of all patients. “However, a meta-analysis has now reconfirmed that a large PFS difference translates into a survival difference as early as 4-5 years, which in turn becomes larger with increasing follow-up time. The survival benefit (10%) of 6 cycles of BEACOPPescalated at 5 years compared with ABVD found in the network meta-analysis is not only supported by the best possible evidence, but also highly clinically relevant,” the speaker concluded.
Source: “CLL and Hodgkin lymphoma”, presentation at OnkoUpdate 2020, 29-30.01.2020 Berlin (D).
InFo ONCOLOGY & HEMATOLOGY 2020; 8(1): 26-27 (published 2/25/20, ahead of print).