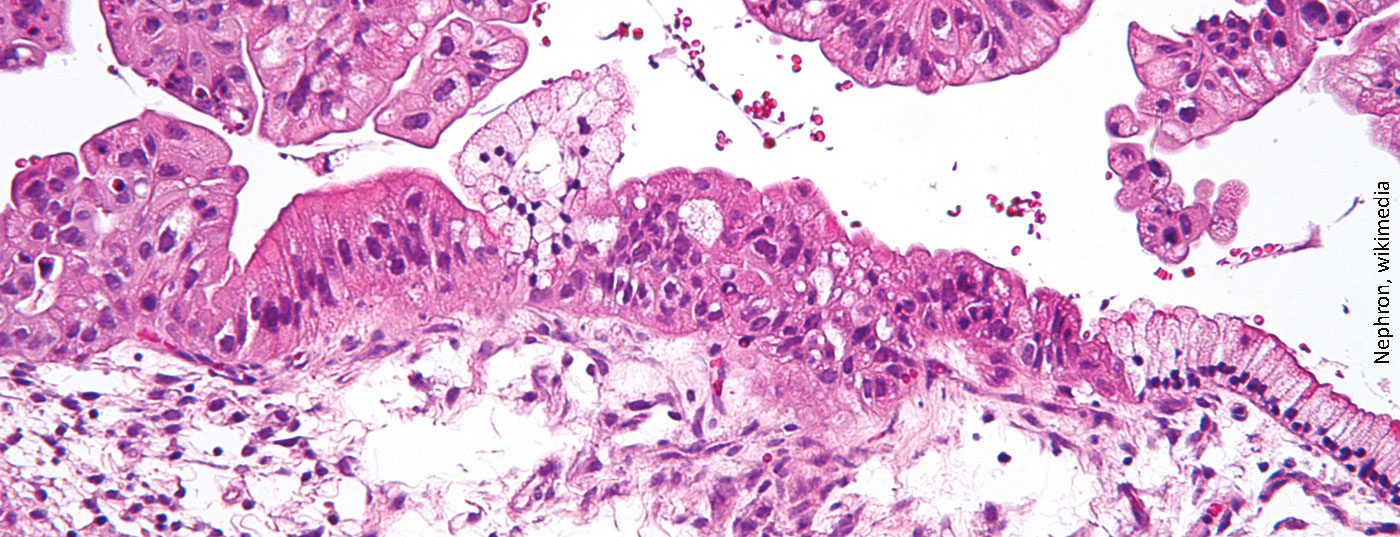
Ovarian cancer is the eighth leading cause of death among women with cancer worldwide. In Switzerland, approximately 620 patients are newly diagnosed with ovarian cancer each year. Approximately 22% of patients with ovarian cancer have a BRCA1/2 mutation. Recent trial data now show that olaparib has a clear long-term benefit over placebo in terms of progression-free survival.
Ovarian cancer is the gynecological tumor with the worst prognosis. The approximately 620 Swiss women who are newly diagnosed with ovarian cancer each year are offset by 416 deaths. For women with BRCA-mutated advanced ovarian cancer, olaparib (Lynparza®) is available as first-line maintenance therapy after platinum-containing chemotherapy has achieved complete or partial remission. Data from the 5-year follow-up have now shown that PARP inhibitor reduced tumor progression/mortality risk by 67% and improved progression-free survival (PFS) to a median of 56.0 months (versus 13.8 months for placebo). After five years, 48.3% of patients treated with olaparib were progression-free, compared with 20.5% on placebo. Median treatment duration was 24.6 months versus 13.9 months. The safety profile was consistent with previous observations. The most common adverse events (AEs) occurring with a frequency of ≥20% were nausea (77%), fatigue/asthenia (63%), vomiting (40%), anemia (39%), and diarrhea (34%). The most common UE of a grade ≥3 were anemia (22%) and neutropenia (9%).
Source: “Lynparza® improved the median time patients with BRCA-mutated advanced ovarian cancer survived without tumor progression to more than four and a half years, compared with just over one year with placebo,” Oct. 12, 2020, AstraZeneca and MSD.
InFo ONCOLOGY & HEMATOLOGY 2020; 8(5): 37.