Interventional therapy of atrial fibrillation is based on trigger elimination and substrate modification. Substrate modification refers to the creation of linear lesions in the left atrium and focal ablation at sites with complex fractionated atrial electrical signals (CFAE). The recommendation that paroxysmal AF be treated by trigger elimination and persistent fibrillation by additional creation of linear lesions is being challenged by new diagnostic methods that can localize and quantify structural and electrical changes in the atria.
Atrial fibrillation is the most common arrhythmia in Western society. Haissaguerre’s reports of focal electrical depolarizations in muscle strands within the pulmonary veins as triggers of atrial fibrillation was the basis for interventional therapy of atrial fibrillation [1]. Using catheter technology and various forms of energy (radiofrequency, cold, laser), these muscle strands are cut from endocardial, isolating the trigger from the atrial myocardium. Pulmonary vein isolation has become the treatment of choice for paroxysmal AF but is increasingly performed for persistent AF. However, the success rate of interventional treatment of atrial fibrillation is not satisfactory. In patients with paroxysmal atrial fibrillation, despite multiple interventions, continuous improvements in technique and method, the success rate decreases from 80% after one year to 60% after five years. Only about 40% of patients with persistent AF are still in sinus rhythm after three years [2]. The underlying mechanisms for atrial fibrillation, especially in persistent atrial fibrillation, are largely unclear. Although recurrences in paroxysmal AF can often be explained by gaps in the ablation line, in a proportion of patients and particularly in patients with persistent AF, other mechanisms are likely to be responsible for the development or maintenance of the arrhythmia.
The substrate describes cellular and ultrastructural changes in the atrial myocardium, which, in addition to the trigger in the pulmonary veins, forms the basis for the development or maintenance of the arrhythmia. Multiple ectopic focal depolarizations, the generation of multiple electrical “waves” propagating heterogeneously across the atrial myocardium, rotors, excitations circling rapidly in a small space, and also functional reentry mechanisms without the presence of structural changes in the myocardium are considered responsible for the maintenance of atrial fibrillation [3–5]. This article presents a review of recent developments to more accurately differentiate structural and electrical changes in atrial fibrillation patients.
Atrial fibrillation – trigger and substrate
The electrophysiological explanation for the development and maintenance of atrial fibrillation is the trigger and the substrate. Atrial fibrillation triggers usually involve muscular extensions of the left atrium into the pulmonary veins [6]. Other focal depolarizations that can be faked as triggers may be found, for example, within the atrial myocardium or in muscle fibers at the junction of the right atrium and the superior vena cava. Clusters of nerve ganglia are often found near the junction of the left atrium with the pulmonary veins and can lead to electrical depolarizations of these muscle fibers, initiating atrial fibrillation. Triggers are also external influences on the autonomic nervous system (e.g. surgery, stress) as well as noxae such as alcohol, which can induce atrial fibrillation.
Fibrosis of the left atrium is causative for the maintenance of atrial fibrillation. However, the definition of this tissue, called substrate, is inconsistent. Underlying the gross description of fibrosis are cellular and ultrastructural changes in the atrial musculature. Chronic atrial fibrillation, in turn, leads to electrophysiological changes, especially a shortening of the refractory period of muscle cells, which in turn promotes the maintenance of atrial fibrillation.
The clinical progression from paroxysmal to persistent and finally to permanent atrial fibrillation should reflect these changes in the atrium. Thus, in patients with paroxysmal atrial fibrillation, spontaneous high-frequency electrical activity in the pulmonary veins causes atrial fibrillation. Once electrical activity in the pulmonary veins ceases, conversion to sinus rhythm occurs. If persistent atrial fibrillation exists, by definition seven days or more, it is referred to as persistent atrial fibrillation and changes in the atrial muscles are assumed to favor the maintenance of fibrillation. More triggers and circular excitations – rotors develop, maintaining atrial fibrillation. During phases of sinus rhythm, these mechanisms are, at least partially, reversed. Atrial fibrillation thus promotes atrial fibrillation and sinus rhythm promotes sinus rhythm [7–9]. On this basis, recommendations were made for catheter-based treatment of AF: pulmonary vein isolation with creation of a circumferential ablation line around both pairs of pulmonary veins for paroxysmal, and additionally linear lesions in the left atrium or ablation of triggers outside the left atrium for persistent and permanent fibrillation. The basis for linear lesions, whereby modification of the substrate is to be achieved, has been adopted from the surgical treatment of atrial fibrillation. In the so-called maze operation, the left atrium is cut and sutured according to a specific pattern. Subsequent scarring, which provided a barrier to electrical impulses, allowed the uncoordinated electrical impulses to be interrupted.
However, based on recent imaging studies in atrial fibrillation patients, it is questionable whether the choice of catheter-based therapy should be based on clinical presentation. Generally accepted is the isolation of the pulmonary veins from the left atrium as a basis for catheter-based intervention. However, who benefits from additional lesions is unclear and is performed very differently in practice. This question is important not least because any creation of a linear lesion increases the risk of the procedure and carries an additional arrhythmic risk.
The atrial fibrillation substrate
Fibrosis of the atrial muscles is considered a hallmark of structural remodeling [10]. The proliferation of fibroblasts alters cell architecture. External influences such as ischemia, oxidative and mechanical stress, and inflammatory stimuli lead to fibroblast proliferation and migration and differentiation into myofibroblasts, which in turn promote fibrosis through production of cytokines and growth factors [11, 12]. Altered cell architecture and myocell-fibroblast interactions alter conduction velocity, resting membrane potential, repolarization, and excitability. An arrhythmogenic substrate with spontaneous depolarizations, emergence of rotors, and reentry mechanisms develops, providing the basis for the maintenance of atrial fibrillation.
Atrial fibrosis is interestingly observed not only in atrial fibrillation patients with structural heart disease but also in those with “lone atrial fibrillation”; there seems to be a correlation between the amount of atrial fibrosis and the persistence of fibrillation [13, 14]. The mechanisms by which atrial fibrosis maintains atrial fibrillation is the subject of intense investigation. It would be interesting for the clinician to know the extent of fibrosis before a catheter-based intervention in order to plan the intervention accordingly (isolation of the pulmonary veins or additional linear lesions depending on the degree of fibrosis or advising against an intervention in case of pronounced fibrosis).
Drug therapy
Drug therapy approaches are based on influencing fibroblast activity or proliferation. In experimental studies, statins and inhibition of the renin-angiotensin-aldosterone system (RAAS) by ACE inhibitors, angiotensin receptor and aldosterone antagonists were able to favorably influence the substrate. However, clinical data are currently insufficient to make general recommendations. However, blockade of the RAAS system, at least in certain patient populations, is an interesting approach in the drug treatment of AF.
Noninvasive assessment of the atrial substrate.
The size of the left atrium allows indirect assessment of the left atrium. Common methods used for this purpose are transthoracic echocardiography and computed tomography or magnetic resonance imaging (MRI). It is generally assumed that there is an increase in atrial fibrosis with increasing left atrial size.
Several studies have demonstrated an increased recurrence rate after catheter-based treatment of atrial fibrillation in patients with an enlarged diameter or volume of the left atrium [15, 17]. Limitations of this method are the availability of a computer tomogram and especially a magnetic resonance tomogram in the practice and the low sensitivity of the method. MRI offers a distinct advantage, namely the imageability of fibrosed tissue, over other methods.
While patients with paroxysmal AF have a majority of low fibrosis and those with persistent fibrillation have a greater fibrosis, left atrial fibrosis is found heterogeneously in both groups. In addition, the recurrence rate after catheter ablation correlates with the degree of fibrosis, and patients with left atrial fibrosis benefit from additional linear lesions. However, fibrosis percentage does not correlate with atrial volume [18]. Our own observations support this approach. Endocardial determination of the electrical voltage of the atrial myocardium (voltage map) showed, on the one hand, that enlargement of the atrium is not necessarily associated with areas of decreased voltage; on the other hand, “low-voltage” areas were found in 20% of paroxysmal atrial fibrillation patients and in persistent atrial fibrillation in only 35% of cases(Fig. 1, unpublished data).
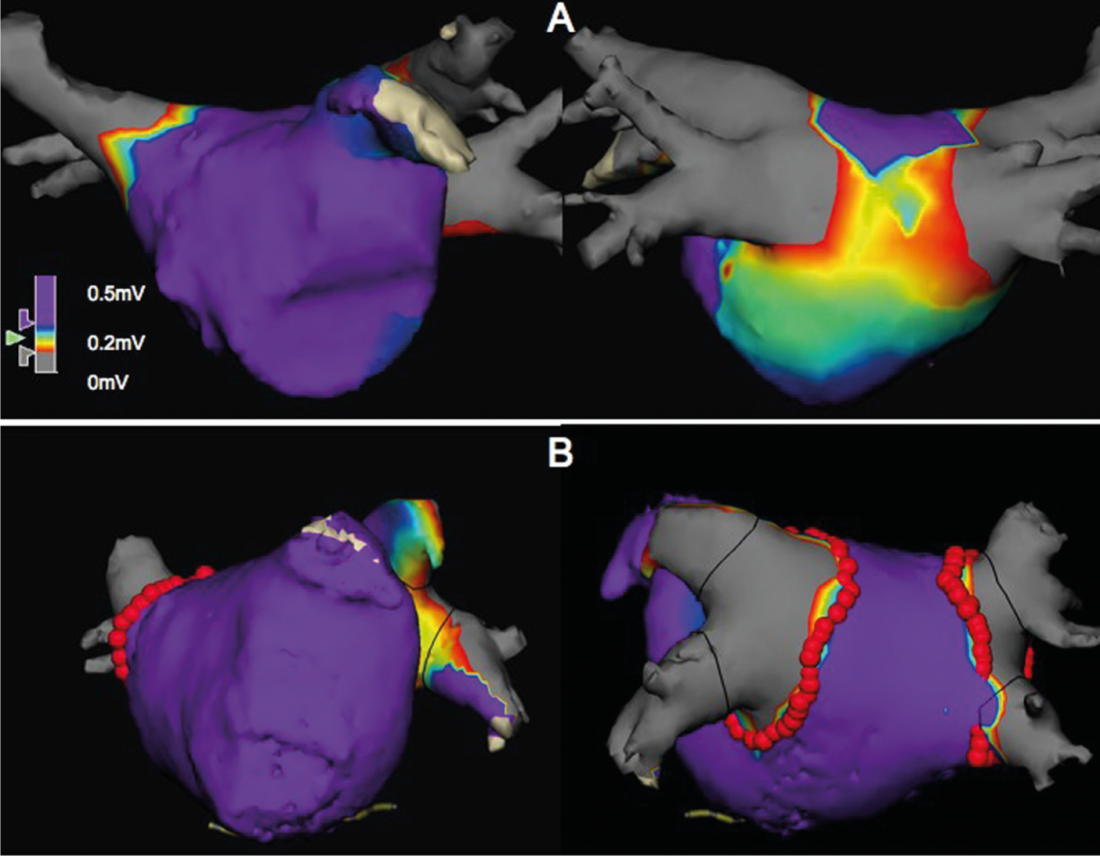
Fig. 1: Bipolar endocardial voltage maps of the left atrium, left antero-posterior, right postero-anterior view, each showing 2 right and left pulmonary veins, respectively, and the atrial ear (antero-posterior view). Areas in purple have an electrical voltage >0.5mV. The green to red area has a voltage of 0.2- 0.5 mV and corresponds to a “low-voltage” area, which is consistent with fibrosis. The upper two images show an extensive “low-voltage” area on the posterior wall in a patient with paroxysmal AF, whereas no “low-voltage” areas were found in the lower images in a patient with persistent AF. The red dots represent the circumferential antral ablation line.
MRI of the atria would allow patients to be counseled about expected chances of success and risks according to the amount of fibrosis, and the interventionalist can better plan the intervention, for example, to avoid linear lesions in persistent AF without relevant fibrosis. However, this method is not yet available for routine use.
High-resolution endocardial mapping of the atria using multipolar catheters has identified stable electrical rotors maintaining fibrillation in atrial fibrillation patients [19]. Rotors and focal triggers, regardless of atrial fibrillation type (paroxysmal, persistent, long-standing), were found in both left and right atria. Radiofrequency ablation of such rotors was significantly more likely to result in acute termination or slowing of AF and showed a higher freedom from recurrence compared with conventional pulmonary vein isolation [20]. This finding suggests that electrical rotors play a critical role in the maintenance of atrial fibrillation.
Interestingly, stable rotors were not found in areas with complex fractionated atrial electrograms (CFAE) [21], ablation of which, in addition to pulmonary vein isolation, is recommended in patients with persistent AF.
Based on the findings of intracardiac mapping, we attempt to translate the highly complex electrical activity during atrial fibrillation to parameters in the surface ECG. It is questionable whether this information can become a noninvasive method for accurate localization and subsequent radiofrequency ablation of such rotors. However, it is possible to imagine statements about number and localization in the right or left atrium, which make an intervention plannable in a certain sense [22].
Another non-invasive method currently being tested is “body surface mapping”. Here, atrial fibrillation activity is measured via a large number (>250) of body surface electrodes. The relative position of the electrodes is then transferred to a computer tomogram of the atria. After processing this electrical information, the number and localization of rotors and focal depolarizations are transferred to the surface of the atria. Initial reports using this method for targeted ablation of triggers and rotors are promising but remain to be tested in larger patient populations [23].
Conclusions
No routinely applicable method is currently available to differentiate the atrial substrate in patients with AF. Previous interventional therapeutic strategies have been based on the assumption of increased fibrosis in persistent AF and the consequent recommendation to produce linear lesions in addition to pulmonary vein isolation. New studies are promising to characterize the localization and quantification of the electrical substrate, rotors, and focal electrical depolarizations to enable targeted ablation.
It is conceivable that catheter-based treatment of AF in the future will be individualized, based on the presence of fibrotic tissue or identification of triggers and rotors.
David Altmann, MD
Literature:
- Haissaguerre M, Jais P, Shah DC, et al: Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339: 659-666.
- Ganesan AN, Shipp NJ, Brooks AG, et al: Long-term Outcomes of Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-analysis. J Am Heart Assoc 2013; 2: e004549.
- Moe GK, Abildskov JA: Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 1959; 58: 59-70.
- Jalife J, Berenfeld O, Mansour M: Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res 2002; 54: 204-216.
- Eckstein J, Verheule S, de Groot NM, Allessie M, Schotten U: Mechanisms of perpetuation of atrial fibrillation in chronically dilated atria. Prog Biophys Mol Biol 2008; 97: 435-451.
- Cheung DW: Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea-pig. J Physiol 1981; 314: 445-456.
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA: Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92: 1954-1968.
- Morillo CA, Klein GJ, Jones DL, Guiraudon CM: Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation 1995; 91: 1588-1595.
- Kaseda S, Zipes DP: Contraction-excitation feedback in the atria: a cause of changes in refractoriness. J Am Coll Cardiol 1988; 11: 1327-1336.
- Burstein B, Nattel S: Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 2008; 51: 802-809.
- Swynghedauw B: Molecular mechanisms of myocardial remodeling. Physiol Rev 1999; 79: 215-262.
- Weber KT, Sun Y, Tyagi SC, Cleutjens JP: Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol 1994; 26: 279-292.
- Boldt A, Wetzel U, Lauschke J, et al: Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 2004; 90: 400-405.
- Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A: Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997; 96: 1180-1184.
- den Uijl DW, Tops LF, Delgado V, et al: Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol 2011; 107: 243-249.
- Berruezo A, Tamborero D, Mont L, et al: Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J 2007; 28: 836-841.
- Shin SH, Park MY, Oh WJ et al. Left atrial volume is a predictor of atrial fibrillation recurrence after catheter ablation. J Am Soc Echocardiogr 2008; 21: 697-702.
- Akoum N, Daccarett M, McGann C, et al: Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol 2011; 22: 16-22.
- Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS: Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol 2012; 23: 1277-1285.
- Narayan SM, et al: Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012; 60: 628-636.
- Narayan SM, et al: Panoramic Electrophysiological Mapping but not Electrogram Morphology Identifies Stable Sources for Human Atrial Fibrillation: Stable Atrial Fibrillation Rotors and Focal Sources Relate Poorly to Fractionated Electrograms. Circ Arrhythm Electrophysiol 2013; 6: 58-67.
- Jones AR, Krummen DE, Narayan SM: Non-invasive identification of stable rotors and focal sources for human atrial fibrillation: mechanistic classification of atrial fibrillation from the electrocardiogram. Europace 2013.
- Haissaguerre M, et al: Noninvasive Panoramic Mapping of Human Atrial Fibrillation Mechanisms: A Feasibility Report. J Cardiovasc Electrophysiol 2012 doi: 10.1111/jce.12075. [Epub ahead of print]