Treatment management for patients with primarily advanced or recurrent endometrial cancer is changing. The potential of dostarlimab, which is particularly effective in patients with mismatch repair deficiency/high microsatellite instability, offers the opportunity to achieve this. However, further interim evaluations also raise hopes for more.
Endometrial carcinoma is the sixth most common cancer in women worldwide and the second most common type of gynecological cancer. In the first-line treatment of primary advanced or recurrent endometrial cancer, carboplatin plus paclitaxel is generally used as standard chemotherapy. However, the long-term results are still poor, with a median overall survival of less than 3 years. Mismatch repair deficient (dMMR) tumors with high microsatellite instability (MSI-H) account for 25-30% of endometrial cancers. The increased expression of programmed cell death receptor 1 (PD-1) and its ligands (PD-L1 and PD-L2) and the high mutational burden of dMMR-MSI-H tumors therefore make them potentially susceptible to anti-PD-1 and anti-PD-L1 therapies. Therefore, the active immune checkpoint inhibitor targeting the PD-1 receptor, dostarlimab, was investigated in more detail for this clientele. The drug was approved in Switzerland based on the results of the GARNET trial for the treatment of adult patients with recurrent or advanced dMMR MSI-H endometrial cancer (EC) that has progressed during or after prior treatment with a platinum-containing regimen. The data also demonstrate durable antitumor activity in patients with mismatch repair-proficient (pMMR), microsatellite-stable (MSS) tumors, although response was less frequent than in patients with dMMR-MSI-H tumors. Because cytotoxic chemotherapy may also have immunomodulatory effects, such as disruption of immunosuppressive signaling pathways and enhanced cytotoxic T-cell responses, the combination of chemotherapy and immunotherapy may have synergistic effects in the tumor microenvironment. Clinical benefits, including improved survival, have been reported with this combination in various cancer types.
Focus on progression-free survival and overall survival
In a randomized, double-blind phase III study, the efficacy and safety of dostarlimab in combination with carboplatin and paclitaxel were analyzed in comparison with placebo plus carboplatin and paclitaxel in 494 patients with primary advanced or recurrent endometrial cancer. Patients were randomized in a ratio of 1:1 ratio, patients received either 500 mg of the monoclonal antibody or placebo intravenously in combination with carboplatin at an area under the curve of 5 mg per milliliter per minute and paclitaxel at a dose of 175 mg per square meter of body surface area intravenously every three weeks for the first six cycles, followed by dostarlimab (1000 mg) or placebo intravenously every six weeks for up to three years or until disease progression, discontinuation of treatment due to toxic effects, patient withdrawal, investigator’s decision to withdraw the patient, or death.
The primary endpoints were progression-free survival (PFS) in patients with primary advanced or recurrent dMMR MSI-H endometrial cancer and in the overall population, and overall survival (OS) in the overall population. Progression-free survival was defined as the time from randomization to the earliest date of radiologic assessment of progressive disease or death from any cause in the absence of progressive disease, whichever occurs first. Overall survival was defined as the time from randomization to death from any cause. Secondary endpoints included progression-free survival determined by a blinded, independent central review, objective response, disease control, duration of response, time to second disease progression, patient-reported outcomes, and pharmacokinetic and immunogenicity analyses.
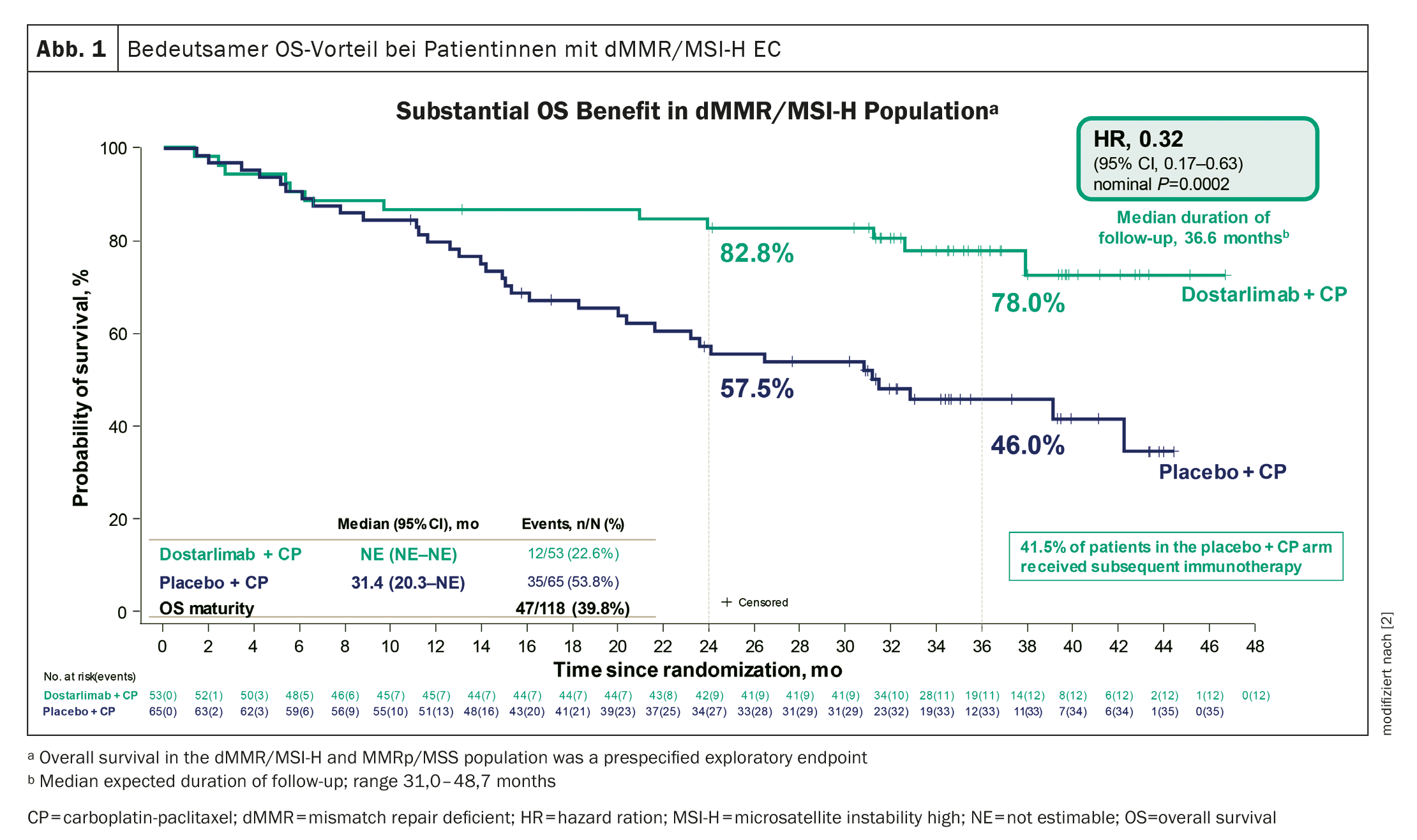
Significant advantage in overall survival
The median duration of follow-up was 24.8 months in the dMMR-MSI-H population and 25.4 months in the overall population.
At the cut-off date, 36% of the dMMR-MSI-H population in the dostarlimab group and 72% in the placebo group had died or had disease progression.
In the overall population, 55.1% in the dostarlimab group and 71.1% in the placebo group had died or had disease progression.
Dostarlimab treatment was associated with a 72% lower risk of disease progression or death in patients with dMMR MSI-H tumors than placebo treatment.
In the overall population, PFS at 24 months was 36.1% in the verum group and 18.1% in the placebo group.
The OS was also longer with the combination treatment with the monoclonal antibody than with placebo at a follow-up time of 25.4 months, but the results did not reach the significance level that was set as the stopping rule.
The latest results of a second interim analysis now paint a more accurate picture.
A significant and unprecedented OS benefit has been demonstrated in patients with dMMR/MSI-H EC (Fig. 1) . In addition, treatment with dostarlimab showed a statistically significant and clinically meaningful OS improvement in the overall population and a consistent benefit in most subgroups.
Subgroups also benefit
While dMMR-MSI-H tumors are predominantly endometrioid, pMMR-MSS tumors are more heterogeneous and include high-risk histological subtypes – including carcinosarcomas. Patients with carcinosarcomas were included in the study. Tumors that are pMMR-MSS generally have a lower tumor mutational burden, but PD-1 expression is prevalent in pMMR-MSS endometrial cancer. A benefit of the dostarlimab** regimen was also observed in the pMMR-MSS population, although it was smaller than in the dMMR-MSI-H population. The benefit of the treatment was consistent in terms of progression-free survival and overall survival.
** Dostarlimab is currently only approved in Switzerland in combination with chemotherapy for the dMMR/MSI-H patient population.
Known safety profile confirmed
The safety profile of dostarlimab-carboplatin-paclitaxel corresponded to that of the individual components of the treatment regimen. The frequency of severe and serious adverse events was about 10% higher with Dostarlimab therapy than with placebo therapy. The frequency with which chemotherapy was discontinued was similar in both groups. The quality of life was also similar in both groups during chemotherapy.
Overall, the combination of dostarlimab, carboplatin and paclitaxel significantly improved outcomes in patients with newly diagnosed primary advanced or recurrent endometrial cancer, with a substantial benefit observed in dMMR MSI-H tumors – but also beyond. The data therefore confirm the combination treatment as the new standard of care for patients with primary advanced or recurrent EC, regardless of mismatch repair status.
Literature:
- Mirza MR, Chase DM, Slomovitz BM, et al.: Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med. 2023 Jun 8; 388(23): 2145–2158.
- Powell MA, Auranen A, Willmott L, et al.: Overall survival in patients with primary advanced or recurrent endometrial cancer treated with dostarlimab plus chemotherapy in Part 1 of the ENGOT-EN6-NSGO/GOG-3031/RUBY trial. Poster. Annual Meeting on Women’s Cancer (SGO), 16–18.03.2024, San Diego (USA).
- Mirza MR, Sharma S, Herrstedt J, et al.: Dostarlimab + Chemotherapy for the Treatment of Primary Advanced or Recurrent Endometrial Cancer: Analysis of Progression-Free Survival and Overall Survival Outcomes by Molecular Classification in the ENGOT-EN6-NSGO/GOG-3031/RUBY Trial. Poster. European Society of Medical Oncology (ESMO), 20–24.10.2023, Madrid (ES).
InFo ONKOLOGIE HÄMATOLOGIE 2024; 12(3): 26–27