Acute bronchitis is associated with considerable economic consequences due to absence from work. A meta-analysis published in 2023 investigated whether treatment with a standardized Pelargonium extract can reduce the time of incapacity for work in adults with acute bronchitis. The database came from four double-blind randomized controlled trials.
In view of the considerable number of annual episodes of acute bronchitis in the working population, a reduction in sickness-related incapacity to work has a significant economic impact [1]. In the meta-analysis by Matthys et al. more than 80% of patients were able to return to work after one week of treatment with EPs® 7630, while half of the patients in the placebo group were still unable to work after the same period [1]. EPs® 7630 is a standardized extract obtained from the root of Pelargonium sidoides and has been shown to reduce the severity of viral upper respiratory tract infections [2]. In Switzerland, EPs® 7630 is approved under the trade name Kaloba® for the treatment of acute bronchitis [14].
Productivity losses due to viral respiratory diseases
Acute bronchitis (AB) is an inflammation of the large lower airways and is often caused by a viral infection [3,4]. Characteristic symptoms include coughing with or without phlegm production, fever, malaise, shortness of breath and wheezing [3]. Even though the majority of AB episodes are uncomplicated, they are still associated with negative effects on everyday activities [5]. In Europe, acute coughs and lower respiratory tract infections are among the main causes of lost working hours in adults and are the most common reason for sickness-related absences from work.
The GRACE study was conducted at primary care centers in 12 European countries and found that 55.6% of working patients who consulted a doctor for an acute cough episode were advised to stay away from work. On average, patients stayed away from work/school for 4 days (SD=5 days) [6,7]. Assuming that 5% of the working population misses an average of 4 days of work/school per AB episode per year, it can be estimated that AB causes an annual economic loss of around 200 missed working days per 1000 employees. Therefore, AB and other acute respiratory infections place a significant economic burden not only on the healthcare system, but also on the economy in general by reducing productivity and lost working time [7–9].
Meta-analysis shows significant benefit of EPs® 7630
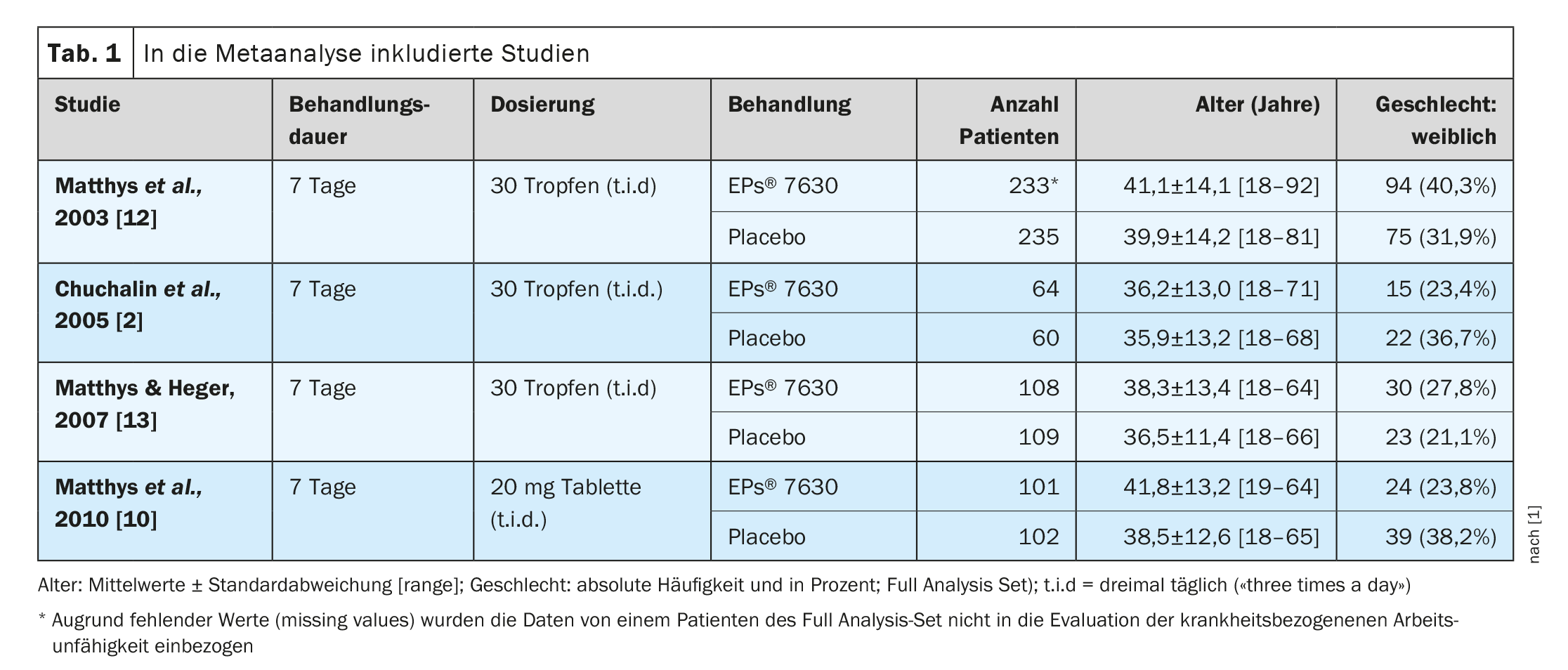
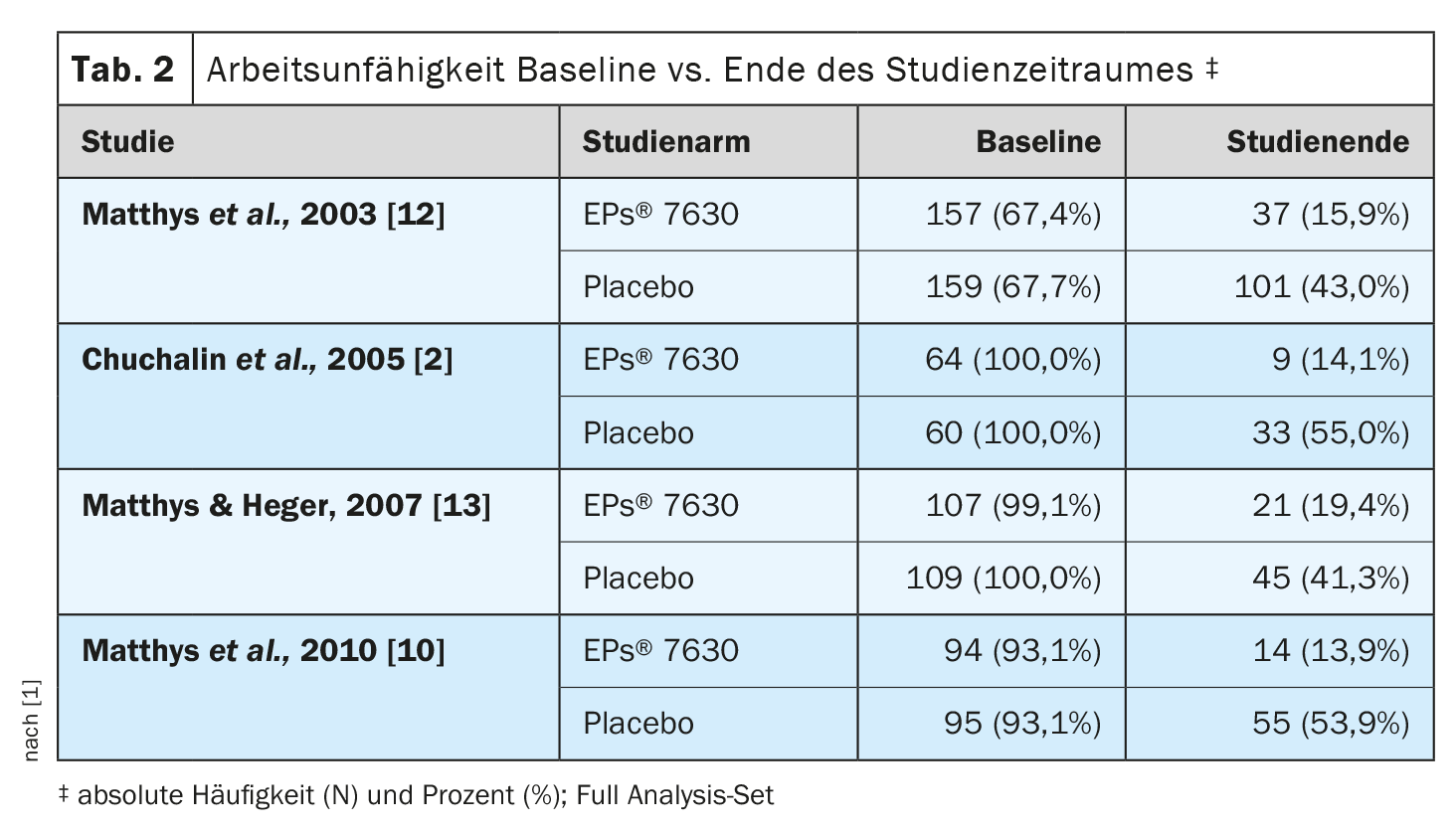
In the meta-analysis by Matthys et al. the average number of days of incapacity for work and the proportion of patients who were still unable to work after one week of treatment were evaluated. Four clinical studies were included with analyzable data from a total of 1011 patients who received the recommended dosage of EPs® 7630 (n=505) or placebo (n=506) for seven days (Table 1) . Three studies investigated EPs® 7630 as a solution, one study used the tablet formulation. In the study by Matthys et al. The study reported in 2010 was a four-arm dose-finding study comparing EPs® 7630 3×10 mg/day, 3×20 mg/day and 3×30 mg/day with placebo [10]. For reasons of comparability, only the marketed dosage of 3×20 mg/day was included in the meta-analysis. According to the study protocols of the eligible studies, the predefined primary efficacy endpoint was the absolute change in total Bronchitis Severity Score (BSS) between baseline and end of treatment on day 7, while work ability was assessed as a secondary endpoint [11]. No clinical studies could be found that examined the patients’ ability to work as a primary endpoint. At baseline, 845 of the 1011 patients were unable to work, corresponding to 83.6%, with the proportion of patients unable to work in the EPs® 7630 group before the start of treatment being similar to that in the placebo group [1] (Tab. 2). At the end of the seven-day treatment period, the proportion of patients unable to work decreased to 14-19% in the EPs® 7630 arm and to 41-55% in the placebo arm across all four studies. A meta-analysis with random effects was also carried out for the proportion of study participants who were still unable to work at the end of treatment. This led to a weighted risk ratio of 0.35 (95% CI: 0.26-0.45; p<0.001) in favor of EPs® 7630. Significant superiority of the herbal medicine over placebo was also observed for each of the four individually assessed clinical trials, with risk ratios ranging from 0.26 to 0.47. A weighted mean difference of 1.73 days (1.17-2.29 days; p<0.001) in favor of EPs® 7630 was found for the number of sick days.
In summary, this meta-analysis shows that a seven-day treatment with Pelargonium sidoides extract EPs® 7630 in adults suffering from acute bronchitis significantly reduces the average number of sick days and significantly increases the proportion of patients who can return to work. From a methodological point of view, the authors point out that the heterogeneity of the studies in this meta-analysis was moderate to low [1].
Literature:
- Matthys H, et al: Effects of EPs 7630 on the duration of inability to work in acute bronchitis – a meta-analysis. Multidiscip Respir Med 2023; 18(1): 914.
- Chuchalin AG, Berman B, Lehmacher W: Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPsR 7630): A randomized, double-blind, placebo-controlled trial. Explore (NY) 2005;1: 437-445.
- Kinkade S, Long NA: Acute bronchitis. Am Fam Physician 2016; 94: 560-565.
- Tanner M, Roddis JK: Antibiotics for acute bronchitis. Nurs Stand 2018; 32: 41-43.
- Verheij T, et al: Acute bronchitis: Course of symptoms and restrictions in patients’ daily activities. Scand J Prim Health Care 1995; 13: 8-12.
- Godycki-Cwirko M, et al: Family practitioners’ advice about taking time off work for lower respiratory tract infections: A prospective study in twelve European primary care networks. PLoS One 2016;11: e0164779
- Hordijk PM, et al: Illness perception and related behavior in lower respiratory tract infections – a European study. Fam Pract 2015; 32: 152-158.
- Birnbaum HG, et al: Economic burden of respiratory infections in an employed population. Chest 2002; 122: 603-611.
- Birnbaum H, et al: Lower respiratory tract infections: Impact on the workplace. Pharmacoeconomics 2003; 21: 749-759.
- Matthys H, et al: Efficacy and tolerability of EPs 7630 tablets in patients with acute bronchitis: A randomized, double-blind, placebo-controlled dose-finding study with a herbal drug preparation from Pelargonium sidoides. Curr Med Res Opin 2010; 26: 1413-1422.
- Lehrl S, et al: The BSS – a valid clinical instrument to measure the severity of acute bronchitis. J Lung Pulm Respir Res 2014; 1: 72-80.
- Matthys H, et al: Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis. A randomized, double-blind, placebo-controlled trial. Phytomedicine 2003; 1: S7-17.
- Matthys H, Heger M: Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): A randomized, double-blind, placebo-controlled, multicentre study. Curr Med Res Opin 2007; 23: 323-331.
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch,(last accessed 08.03.2024)
FAMILY PHYSICIAN PRACTICE 2024; 19(3): 30-32