Personalized therapy is also arriving more and more in diabetes treatment. Phenotyping and closed-loop systems are two buzzwords that provide an important basis for effective therapy.
Prof. Andreas Fritsche, MD, Institute of Diabetes Research and Metabolic Diseases of Helmholtz Zentrum München at the University of Tübingen, spoke about subphenotypes of type 2 diabetes as a future basis for therapy decisions [1]. The limitation to the WHO classification into diabetes type 1, type 2, type 3 (genetic defects, exocrine pancreas disease, endocrinopathies, medications), and gestational diabetes does not do justice to the approach of personalized medicine and is in a state of flux, said the speaker [1].
C-peptide glucose quotient as a differential diagnostic marker.
Regarding the distinction between insulin-dependent type 1 and non-insulin-dependent type 2 diabetes, Prof. Fritsche cautions that this can sometimes be difficult, “but extremely important in today’s world as we increasingly give SGLT-2 inhibitors, which are associated with ketoacidosis risk.” The speaker illustrated that age can be a misleading criterion with data published in the Lancet in 2018 [2], which showed that there is a cumulative increase in type 1 diabetes over six decades of life. Forty-two percent of new cases of type 1 diabetes do not manifest until after the age of 30, and in isolated cases it occurs even at ages older than 75, the speaker said. The fact that the incidence of type 2 diabetes is very high after a certain age can lead to a bias that causes these individual cases to be overlooked or ignored. misdiagnosed. The C-peptide glucose quotient is considered a reliable diagnostic marker. “It’s a simple measure to get therapy safety,” the speaker explained. If the C-peptide glucose ratio is <2, it is type 1 diabetes and insulin therapy tends to be indicated (caveat: discontinue insulin); if it is <1, it is always indicated. GAD antibodies, on the other hand, are not suitable as diagnostic markers due to their lack of sensitivity and specificity.
Subphenotyping of adult onset diabetes
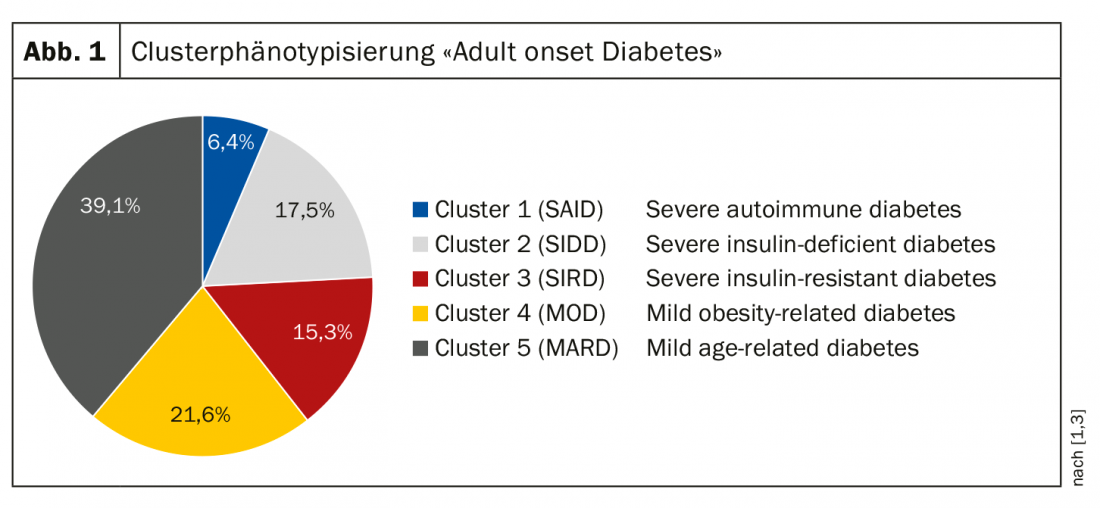
In 2018, Ahlqvist and colleagues published results of a data-based cluster typing [3], which resulted in the following five clusters. (Fig. 1): Cluster 1=SAID (“Severe Auto Immune Diabetes”): 4-6%; Cluster 2=SIDD (“Severe Insulin Dependent Diabetes”): 17.5%; Cluster 3=SIRT (“Severe Insulin Resistant Diabetes”): 15.3%; Cluster 4=MOD (“Mild Obese Diabetes”): 21.6%; Cluster 5=MARD (“Mild Age Related Diabetes”): 39,1%. Correlative relationships between the individual clusters and diabetes-relevant parameters were shown with implications for prognosis and therapy. Cluster 1 and cluster 2 have the highest HbA1c levels and the poorest insulin secretion, and cluster 3 is the most insulin resistant. The insulin-resistant phenotype (cluster 3) has the highest rate of development of chronic renal failure. In terms of therapy, cluster 2 takes the longest to reach the HbA1c target with maximal oral therapy, meaning that these patients are unlikely to be well treated with triple oral therapy and will continue to require insulin. Data from the ADOPT trial published in 2019 [4] were able to replicate this clustering and showed that different clusters show different response to medication (metformin, sulfonylureas, glitazones). Cluster 2 responds very well to glitazones, and sulfonylureas work best in cluster 5. In light of these findings, a change in thinking is called for so that the appropriate antidiabetic is determined based on pathophysiologic criteria, although these new data have not yet been incorporated into the current EASD/ADA guidelines, the speaker said.
“Time in Range” – new target in diabetes therapy
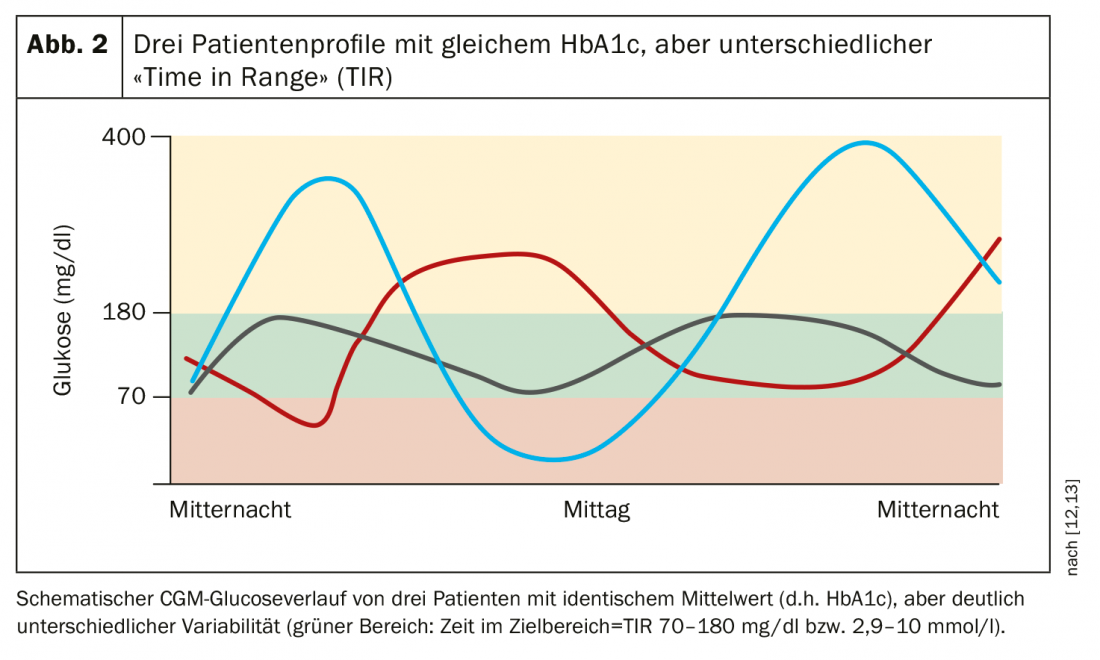
Dr. med. Jens Kröger from the DDG Center for Diabetology Hamburg presented current findings on continuous glucose monitoring systems (CGM) and “Time in Range” (TIR) [5,12]. With the increasing prevalence of continuous glucose monitoring systems (CGM and “flash glucose monitoring”), time on target (TIR) can be easily recorded. In addition to the HbA1c, the TIR is an important additional patient-relevant information for describing the stability of the current glucose control as a basis for therapy decisions, which are not or only insufficiently reflected in the HbA1c (Fig. 2). For example, no statements about glucose stability or variability and relevant hyper- or hypoglycemia are possible on the basis of the HbA1c value. In addition, there is also a problem of bias due to glucose-independent influences (hemoglobinopathies, anemia, renal insufficiency, liver cirrhosis, ethnic differences in glycation rates, interindividual variation in red blood cell survival, etc.) and rapid changes in daily glycemic control are not reflected.
Real time (rt)-CGM systems represent a milestone for type 1 and type 2 diabetes sufferers with regard to therapy management in intensified insulin and insulin pump therapy. Intermittently scanned (Isc)-CGM systems (“Freestyle libre” device) [5] are useful in temporary use with regard to lifestyle intervention and drug optimization in the field of prevention and oral antidiabetic medication in type 2 diabetes. The use of CGM systems resulted in better control of glucose levels/HbA1c values, reduction of hypoglycemia, and limitation of glucose variability [5]. A meaningful reduction in time in hypoglycemia was demonstrated in the IMPACT trial for type 1 diabetes and in the REPLACE trial for type 2 diabetes [6,7]. Structured education programs (SPECTRUM, flash) are the foundation of successful diabetes therapy with continuous glucose monitoring systems [5,8].
Hybrid Closed Loop Systems
Elmar Jaeckel, MD, from Hannover Medical School, Germany, provided an update regarding rtCGM in closed-loop systems [9]. A continuous glucose monitoring (CGM) system includes three components: the wearable sensor, the transmitter, and the receiver [10]. Hybrid closed-loop devices are an evolution based on this: the MiniMed 670G hybrid closed-loop system from Medtronic automatically measures glucose levels in subcutaneous adipose tissue fluid and adjusts insulin delivery to match glucose levels [11]. In the USA, this system has been approved for people aged 14 and older with type 1 diabetes since 2016. “Hybrid” means that not everything is automated yet, for example, the patient still has to enter the carbohydrate intake at meals. In addition, a blood glucose determination for regular calibration and the change of sensor and infusion set is required. The system measures glucose concentration at 5-minute intervals and automatically delivers a dose of insulin adjusted to the value or interrupts insulin delivery. The system consists of a sensor that measures glucose concentration, an insulin pump, and an infusion set that connects the pump to the body. A smartphone or separate CGM device is not necessary.
In addition to improving quality of life by eliminating the need for constant manual monitoring of blood glucose and administration of insulin, a scientific evaluation of hybrid closed-loop systems also showed positive glycemic effects [11]: decrease in HbA1c from 7.7% to 7.1% (adolescents) and from 7.3% to 6.8% (adults), respectively, and an increase in TIR from 60.4% to 67.2% (adolescents) and from 68.8% to 73.8% (adults), respectively.
Real world long-term data is pending at this time as these are relatively new technology products. To date, these have not yet achieved the metabolic control of a biological beta cell substitute [9]. There is also no conclusive clarity regarding the question of which is the right patient for which application.
Source: DGIM 2019, Wiesbaden (D)
Literature:
- DGIM: Prof. Dr. med. Andreas Fritsche, Medical Clinic IV, University Hospital Tübingen, slide presentation: Highlights Diabetology: Subphenotypes of Type 2 Diabetes – Future Basis for Therapy Decision. 125. Congress of the German Society of Internal Medicine, Wiesbaden, May 6, 2019.
- Thomas NJ, et al: Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. The Lancet Diabetes & Endocrinology, 6 (2): 122-129. www.thelancet.com/journals/landia/article/PIIS2213-8587(17)30362-5/fulltext
- Ahlqvist E, at al: Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. The Lancet Diabetes & Endocrinology 2018; 6(5): 361-369.
- Dennis JM, et al: Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes & Endocrinology 2019; 7(6): 442-451.
- DGIM: Jens Kröger, MD, Specialist in Internal Medicine and Diabetology/Diabetologist DDG, Center for Diabetology Hamburg Bergedorf, slide presentation: Highlights Diabetology: CGM and FGM. 125. Congress of the German Society of Internal Medicine, Wiesbaden, May 6, 2019.
- Bolinder J, et al: Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016; 388: 2254-2263. doi: 10.1016/S0140-6736(16)31535-5. epub 2016 Sep 12.
- Haak T, et al: Flash Glucose-Sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-Treated Type 2 Diabetes: a Multicenter, Open-Label Randomized Controlled Trial. Diabetes Ther 2017; 8(1): 55-73. doi: 10.1007/s13300-016-0223-6. epub 2016 Dec 20.
- Center for Diabetology Bergedorf: CGM Training (Spectrum). www.diabeteszentrum-hamburg-ost.de/cgm_schulung_spectrum_de_254.html, last accessed 04 Jun. 2019.
- DGIM: Dr. E. Jaeckel, Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, slide presentation: Highlights diabetology: outlook on the use of rtCGM in closed-loop. 125. Congress of the German Society of Internal Medicine, Wiesbaden, May 6, 2019.
- Klonoff DC, Ahn D, Drincic A: Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract 2017; 133: 178-192.
- Garg SK, et al: Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther 2017; 19(3): 155-163. doi: 10.1089/dia.2016.0421. epub 2017 Jan 30.
- Danne T, et al: Time in Range. New target in the treatment of people with diabetes mellitus. German Health Report Diabetes 2019; 201-207. www.deutsche-diabetes-gesellschaft.de, last accessed 04 Jun 2019.
- DDG: Statement of the Commission Laboratory Diagnostics in Diabetology of the DDG and DGKL On the use of the “Time in Range”: Alternative or useful supplement to the HbA1c? www.deutsche-diabetes-gesellschaft.de, last accessed 04 Jun. 2019.
HAUSARZT PRAXIS 2019; 14(7): 28-29 (published 7/12/19, ahead of print).











