The use of herbal and complementary medicine products is widespread worldwide and is often used in combination with conventional medical therapies. In this article, we report a case of severe herbal-induced liver injury (HILI ) caused by chronic consumption of green tea and a protein shake.
(red) Liver cell damage caused by drugs (DILI, Drug-Induced Liver Injury) is a significant medical problem as it can lead to morbidity and mortality. Hepatotoxicity caused by drugs or herbal products can lead to serious liver dysfunction, which in the worst case can cause liver failure. Particularly problematic are the so-called type II lesions, which occur “unpredictably” and independently of the dose or duration of use and are only observed in predisposed individuals. In such cases, liver toxicity often remains undetected until significant damage has occurred.
Phytoproducts are often seen as natural and therefore harmless alternatives to conventional medicines. This perception, coupled with the lack of rigorous regulatory control of many herbal products, leads to an increased risk of adverse drug reactions (ADRs). In recent years, reports of phytotherapy-induced liver injury (HILI) have increased, particularly with products marketed for weight loss or as dietary supplements. Green tea and branched-chain amino acids (BCAA) are among the products that have been linked to hepatotoxicity.
Case Report
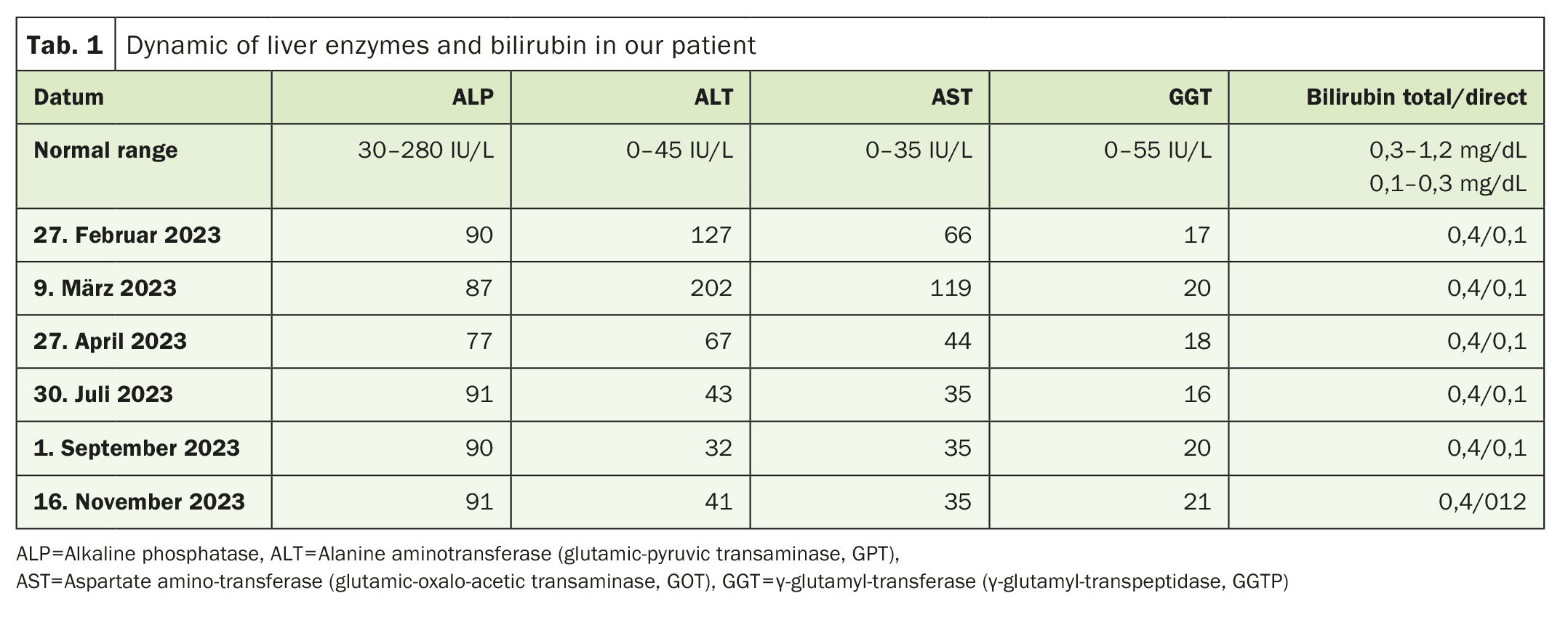
A 39-year-old man, previously considered healthy and not taking regular medication, was referred for further investigation due to elevated liver enzymes. Two months earlier, he had started taking a daily dietary supplement containing green tea and BCAA, together with a protein shake. The patient did not consume excessive amounts of alcohol or sugary drinks. A physical examination and an ultrasound examination of the abdomen revealed no abnormalities. However, the liver enzyme values (ALT, AST, GGT) were significantly elevated, as shown in Table 1.
After consulting with the patient, we recommended stopping the dietary supplements immediately. Over time, liver enzymes dropped to normal levels. A liver biopsy was not performed and the patient refused to resume taking the products to check the reaction. Instead, a lymphocytic toxicity assay (LTA) was performed to confirm the toxic effect of the products.
Methods
The lymphocytic toxicity assay (LTA) is an in vitro method used to evaluate the toxic effect of substances on mitochondria. In this case, the patient’s lymphocytes were incubated after isolation with the products in question (green tea, protein shake and their combination). The MTT test (reduction of a yellow tetrazolium salt to purple formazan) was used to determine the activity of mitochondrial succinate dehydrogenase (SDH) as a measure of cell viability. The toxicity of the products was expressed as a percentage decrease in mitochondrial activity relative to the control group (without product).
In addition, we examined the patient’s immunological profile, in particular the production of proinflammatory cytokines and chemokines. The patient’s serum samples were analyzed for cytokines such as IL-1β, IL-8, IL-15, TNF-α and VEGF, which are typically elevated in cases of hypersensitivity reactions. An ELISA (enzyme-linked immunosorbent assay) was used to quantify these proteins.
Results
The LTA results showed a significant reduction in mitochondrial activity in the patient’s lymphocytes, indicating mitochondrial toxicity. It was particularly striking that the combination of green tea and protein shake showed a stronger toxic effect than the products alone. This indicates that the combination of the two products had a synergistic toxic effect on the mitochondria.
Immunologically, the patient’s serum samples showed significantly elevated levels of proinflammatory cytokines. IL-1β was elevated 1.8 times the upper normal value, while IL-8 and IL-15 reached 2-fold. VEGF was even elevated 10-fold, indicating a massive inflammatory response and potential damage to the vasculature of the liver. Markers of cell damage showed that the majority of liver cell damage was caused by necrosis and not apoptosis, as evidenced by elevated ccK8 (M65) levels.
This case report shows the potentially serious consequences of taking phytoproducts in combination with dietary supplements. While green tea and protein shakes are considered relatively safe individually, their combination can trigger a toxic overreaction in susceptible individuals, leading to severe liver damage. In this case, the combination of mitochondrial dysfunction and a strong immunological response led to pronounced hepatotoxicity.
The increased expression of proinflammatory cytokines, in particular TNF-α and TRAIL, indicates liver cell damage caused by immune activation. This type of reaction is typical of idiosyncratic drug reactions (iDILI) in which genetic or immunological predispositions play a role. The patient showed a pronounced immune reaction to the products, which could be detected by the LTA test even months after exposure.
Another important issue is the lack of regulation of many herbal food supplements. As these products are often classified as foods or dietary supplements, they are not subject to the same stringent testing as conventional medicines. As a result, potential risks to liver health are often not recognized until serious damage occurs. Given the increasing popularity of such products, it is important that both healthcare professionals and consumers are aware of the potential risks.
Conclusion
The case described here underlines the need for increased monitoring and reporting of phytotherapy-induced liver injury. Despite the increasing popularity of herbal remedies, their potential risk of liver damage should not be underestimated. In particular, the combination of products such as green tea and protein shakes can lead to severe liver damage in certain cases. International standards for the assessment and reporting of HILI cases are essential to raise awareness of the potential risks and improve patient protection.
Source: Malnick S, Abdullah A, Maor Y, Neuman MG: Phytotherapy-Induced Hepatocytotoxicity: A Case Report. Curr Issues Mol Biol. 2024 Jul 16; 46(7): 7548-7557. doi: 10.3390/cimb46070448. PMID: 39057089; PMCID: PMC11275310.
PHYTOTHERAPY PRACTICE 2024; 1(1): 12-13