After stent implantation, stent thrombosis is one of the most important acute complications. Effective platelet aggregation inhibition is therefore indicated. But what does this look like?
In the early days of coronary angiography, lesions were initially treated by balloon dilatation alone. It was not until the mid-1980s that the first stents were developed [1,2]. Since then, the technology of stents but also the possibilities in the field of platelet aggregation inhibition have steadily evolved.
Role of dual platelet inhibition (DAPT) and its development.
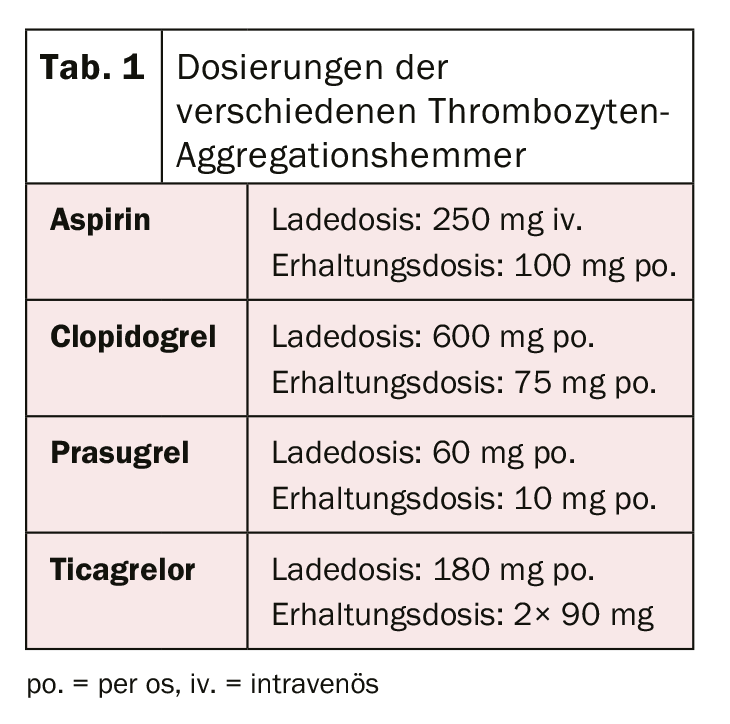
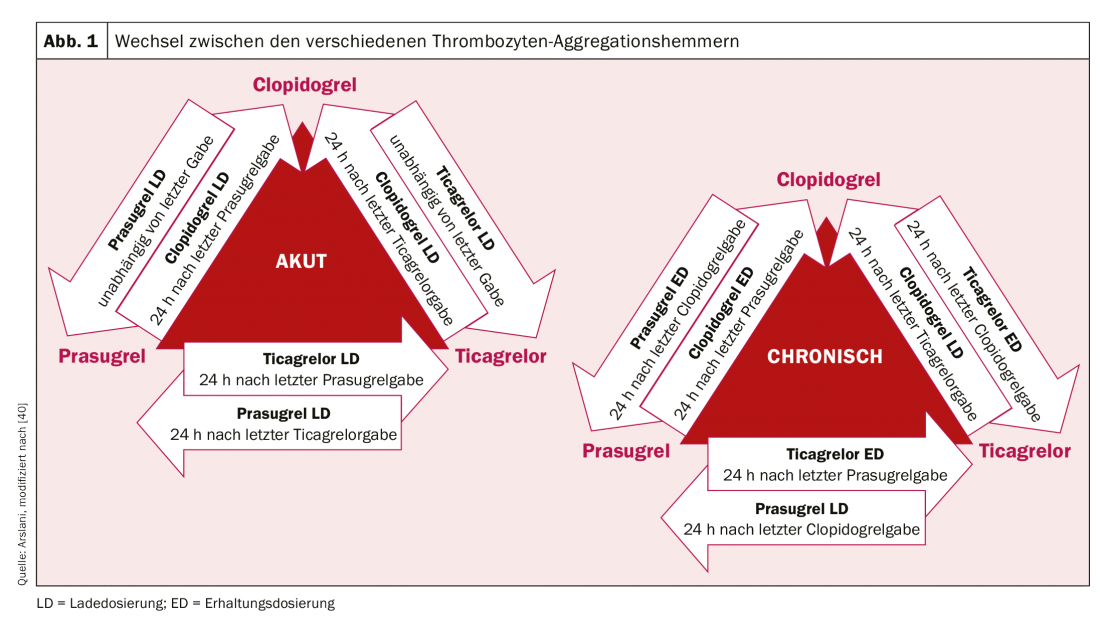
After the revolution of the first stent implantation in 1986 by Sigwart et al. [2], the first associated difficulties and complications became apparent relatively quickly. The most important acute complication after stent implantation is and remains stent thrombosis, which occurred at a rate of almost 20% in the early days. To prevent this, various therapies for blood thinning or platelet aggregation inhibition after stent implantation have been evaluated. After treatment with low-molecular-weight dextran, aspirin (ASA), dipyridamole, and intravenous heparin failed to produce the desired results, patients were systematically anticoagulated with oral warfarin after stent implantation. Although this resulted in a significant reduction in acute and subacute stent thrombosis to 3.5%, it was also associated with a significant increase in bleeding complications [3–5]. In the search for the ideal balance between prevention of the dreaded stent thrombosis and the increased risk of bleeding, various other therapeutic regimens were subsequently investigated. Among these, double (dual) platelet aggregation inhibition appeared to be the most effective. For example, a study by Leon et al. showed that a significant reduction in the risk of stent thrombosis and associated complications could be achieved by combination therapy with ASA and ticlopidine [6,7]. Because severe side effects occurred with ticlopidine (including allergies, ulcers, diarrhea, hepatic dysfunction, and neutropenia), this drug was quickly replaced by the much better tolerated clopidogrel [8]. ASA prevents thromboxane-mediated and clopidogrel prevents ADP-mediated platelet activation, with both blockages acting additively, resulting in significantly more effective inhibition and thus reduction of thrombus formation than monotherapy. Clopidogrel is a prodrug that is first metabolized in the liver by cytochrome P450 2C19 and other CYP enzymes, and its active metabolite subsequently binds irreversibly to platelet P2Y12 receptors, thereby decreasing ADP-mediated platelet activation. Because the enzymes that activate clopidogrel in the liver vary genetically, the platelet-inhibitory effect of clopidogrel is not equally pronounced in all people. After patients underwent elective stenting, the efficacy of dual antiplatelet therapy with ASA and clopidogrel was also demonstrated in patients with acute non-ST elevation myocardial infarction by a significant reduction in cardiovascular events [9]. Further optimization of platelet aggregation inhibition was achieved by the use of new P2Y12 receptor inhibitors. Prasugrel is also a prodrug from the group of thienopyridines, is also metabolized (or activated) in the liver and also binds irreversibly, but faster and more strongly, to the said receptors. In addition, unlike clopidogrel, there are no genetically determined differences in the activation of prasugrel. The TRITON-TIMI 38 trial [10] demonstrated that prasugrel resulted in a further reduction in cardiovascular events compared with clopidogrel. Despite increased bleeding complications, clinical benefit was clearly demonstrated in favor of prasugrel with a significant reduction in ischemic end points (except in patients with age ≥75 years, low body weight (<60 kg), and in patients after cerebrovascular events). In addition, because of the higher risk of bleeding, coronary status should be known or the intention for stent implantation should be present prior to prasugrel administration. Preinterventional loading is currently recommended only for ST-segment elevation myocardial infarction [11]. Ticagrelor belongs to the class of cyclopentylriazolopyrimidines and is a competitive and thus reversible P2Y12 inhibitor, which is taken as an active metabolite (without enzymatic activation). In the PLATO study [12], ticagrelor was also shown to be superior to clopidogrel. A reduction in cardiovascular death, myocardial infarction and stent thrombosis was shown and there was no evidence of increased bleeding complications. However, different bleeding definitions were used in some cases compared with other studies. In contrast to prasugrel, the beneficial effect was also seen in patients treated conservatively (without intervention and stenting) or by bypass surgery [12,13]. In patients with ST-elevation myocardial infarction, pretreatment with ticagrelor before coronary angiography has also been shown to result in lower rates of stent thrombosis [14]. Unfortunately, a comparison between the substances ticagrelor and prasugrel has not yet been performed. The initial loading dose and the maintenance dose of the various drugs are summarized in Table 1. In everyday clinical practice, it may be necessary (for example, in the case of drug intolerance) to switch between preparations. The algorithm for switching between preparations according to clinical setting (chronic versus acute) are shown in Figure 1 .
Stent development
As mentioned earlier, in the early days of coronary intervention, the vessel was treated only by balloon dilatation. However, due to dissection, elastic recoil from overdistension, and late remodeling with intimal proliferation, this often resulted in renewed vessel occlusion and restenosis. To counteract this and to keep the vessels open after dilatation, balloon or self-expandable vascular meshes (stents) were developed, which were implanted after pretreatment by balloon dilatation. Initially, these stents were difficult to use due to their nature, the material used, as well as the still young implantation technique (mostly stainless steel with thick stent struts). Over time, however, these metal stents (now also known as bare metal stents, BMS) evolved to become much easier to use (implantation via balloon expansion, thinner stent struts, lighter alloys). Early on, although BMS was shown to be superior to simple balloon dilatation [3,4], the long-term course showed relevant rates of instent restenosis due to excess intimal proliferation. To counteract this proliferation, the so-called drug-eluting stents (DES) were developed. The first generation DES were made of the same alloys as the BMS but were coated with a polymer-delivered antiproliferative drug such as sirolimus or paclitaxel. This led to a reduction in neo-intima formation and consequently to a significant decrease in instent restenosis [15–17]. However, due to delayed endothelialization caused by the antiproliferative drugs and thus prolonged contact of the stent struts with the bloodstream, several studies showed a significantly increased rate of late stent thrombosis and myocardial infarction [18,19]. In response, not only platelet aggregation inhibition but also stent composition has been further developed. In the second and third generation DES, biocompatible polymers and more efficient drugs, such as everolimus, have been used for coating at lower doses, resulting in faster and better endothelialization of the stents. Together with the improved DAPT, the DES in use today have been shown to significantly improve safety while maintaining effectiveness compared to first-generation DES [20,21], which has led to the virtual disappearance of uncoated BMS. In a further step, in addition to the new stents, balloon dilatation has now evolved and there is the possibility to treat smaller vessels using drug-coated balloons (DCB) without stent implantation [22].
Duration of DAPT after stent implantation
Regarding the duration of DAPT, a clear distinction should be made between patients with acute myocardial infarction and patients with stable coronary artery disease. In patients with stable coronary artery disease, it has been shown that there is no difference in cardiovascular events and death between a treatment duration of 6 months and ≥12 months [23,24]. On the other hand, a significant increase in relevant bleeding complications occurred with a treatment duration of more than 12 months and there was evidence for increased mortality [25,26]. Because no trials of DAPT consisting of ASA and ticagrelor or prasugrel have been performed to date in patients with stable coronary artery disease, DAPT with clopidogrel is recommended in this setting. However, in selected patients at high ischemic risk, one of the other drugs may be chosen. In conclusion, dual antiplatelet therapy with ASA and clopidogrel for 6 months is recommended in patients with stable coronary artery disease after intervention, regardless of stent type, and also after treatment with a DCB alone [27]. If there is an increased risk of bleeding (Precise-DAPT score ≥25, www.precisedaptscore.com) [28], the duration of therapy can be reduced to 3 months [29,30] and in selected cases to 1 month [31,32]. However, when reducing the duration of therapy (especially when reducing to 1 month), the risk of recurrence of cardiovascular events must be carefully weighed against the risk of bleeding.
Recommendations for patients after an acute coronary syndrome are based on the comparative studies of prasugrel or ticagrelor with clopidogrel. These studies are the basis for the current recommendation that patients should receive dual antiplatelet therapy with ASA and ticagrelor or prasugrel for 12 months after an acute event, also regardless of stent type [9,10,12]. According to current data, in particular the PEGASUS study. [33] Prolonged dual antiplatelet therapy should be used only in patients at increased ischemic risk (age ≥50 years and more than one of the following: >65 years, diabetes mellitus requiring treatment, further myocardial infarction, multivessel coronary disease, or renal insufficiency with a clearance of <60 ml/min) and without previous bleeding complications should be considered. Therapy with ticagrelor 60 mg twice daily is preferred [27]. Reducing the duration of therapy to 6 months is also possible with an acceptable risk if the bleeding risk is elevated (Precise-DAPT score ≥25) [28]. In the data to date, only a reduction to <6 months showed a substantial increase in cardiovascular events.
Anticoagulation and DAPT
Patients with an indication for oral anticoagulation present another challenge. Again, the ideal balance between bleeding risk and the risks of stent thrombosis or cardiovascular events must be found. Triple therapy with ASA, clopidogrel, and established oral anticoagulation with either a vitamin K antagonist (VKA) or one of the new oral anticoagulants (NOAK) is recommended. The target INR value should be chosen rather at the lower limit (INR 2-2.5) and for NOAKs the lowest approved dosage for the prevention of cerebrovascular events should be chosen (dabigatran 110 mg 2× daily, rivaroxaban 15 mg 1× daily, apixaban 5 mg 2× daily or 2.5 mg if 2 of the following risk factors apply: age ≥80 years, weight ≤60 kg, creatinine ≥133 umol/l). [27]. Combined oral anticoagulation with ASA and clopidogrel [34], as there is little data regarding the other P2Y12 inhibitors and an increased risk of bleeding has been described in registry data [35]. In determining the duration of triple therapy, the risk of bleeding (HAS-BLED score [hypertension, abnormal renal/liver function, history of stroke, history of bleeding, unstable INR setting, age over 65 years, alcohol abuse/medications e.g., NSAIDs; high risk of bleeding with ≥3 points) [36] must again be weighed against the risk of thrombosis. If the risk of bleeding is high, triple therapy should be limited to one month and then dual therapy (ASA or clopidogrel + oral anticoagulation at normal doses) should be continued for a total of one year [34,37]. If the risk of thrombosis is high, an extension of triple therapy up to 6 months is recommended [38], after which dual therapy with ASA or clopidogrel should also be followed for another 6 months. After one year, monotherapy with oral anticoagulation can then be continued in most cases [39].
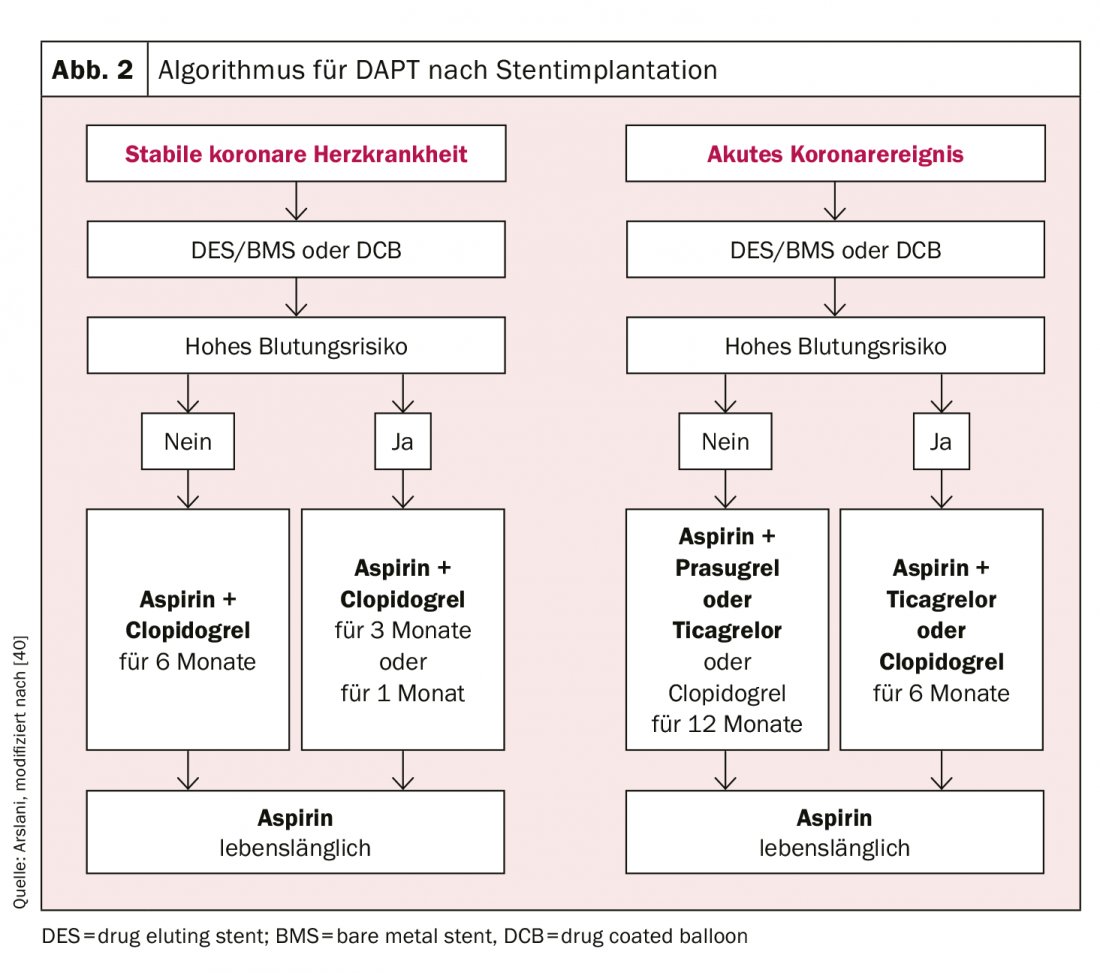
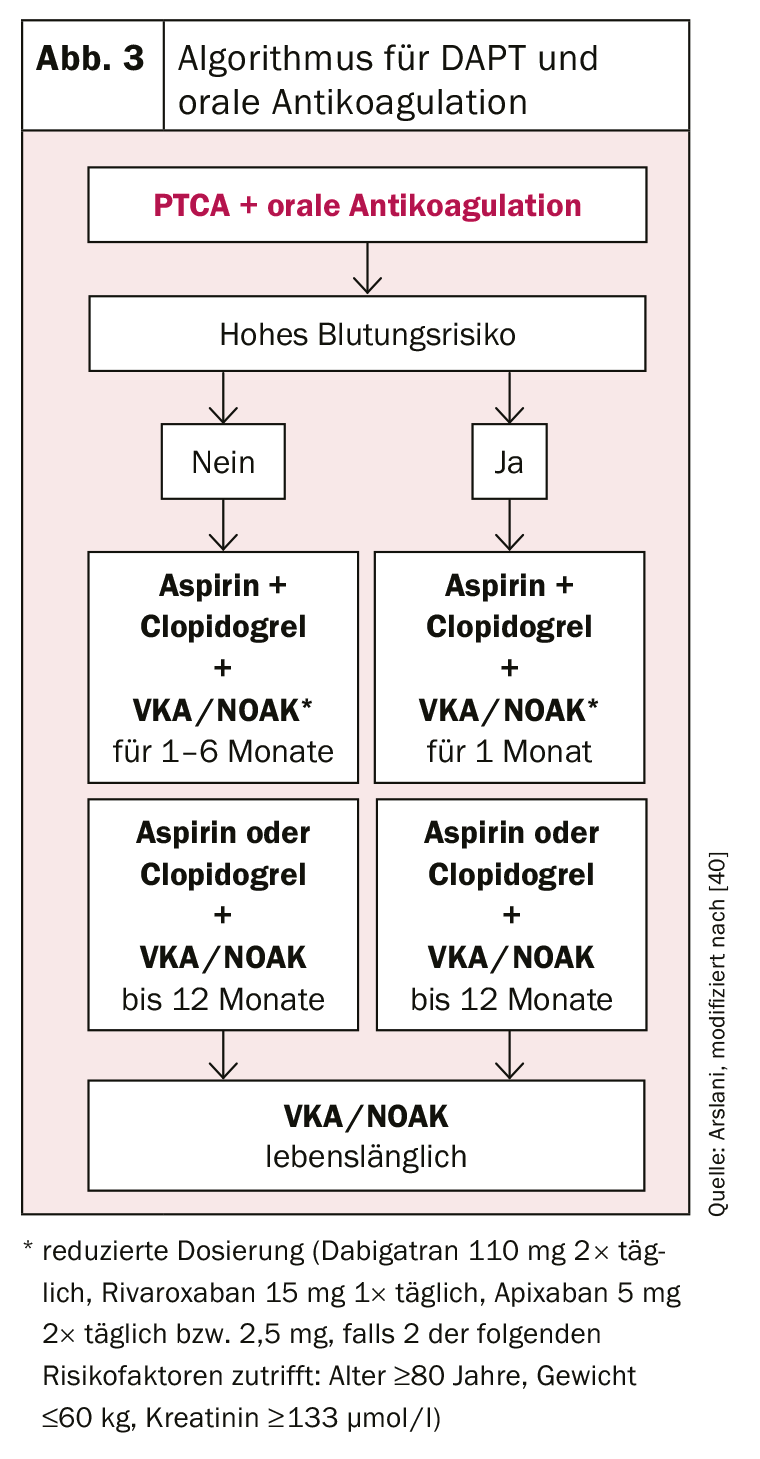
The use of the various antiplatelet agents according to the current recommendations of the European Society of Cardiology are summarized in Figure 2 and 3 .
Literature:
- 1 Gruntzig A: Transluminal dilatation of coronary-artery stenosis. Lancet, 1978. 1(8058): 263.
- 2 Sigwart U, et al: Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med, 1987. 316(12): 701-706.
- Fischman DL, et al: A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med, 1994. 331(8): 496-501.
- Serruys PW, et al: A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study GrouN Engl J Med, 1994. 331(8): 489-495.
- Cohen DJ, et al: In-hospital and one-year economic outcomes after coronary stenting or balloon angioplasty. Results from a randomized clinical trial. Stent Restenosis Study Investigators. Circulation, 1995. 92(9): 2480-2487.
- Leon MB, et al: A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med, 1998. 339(23): 1665-1671.
- Schomig A, et al: A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med, 1996. 334(17): 1084-1089.
- Bertrand ME, et al: Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation, 2000. 102(6): 624-629.
- Yusuf S, et al: Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med, 2001. 345(7): 494-502.
- Wiviott SD, et al: Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med, 2007. 357(20): 2001-2015.
- Montalescot G, et al: Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med, 2013. 369(11): 999-1010.
- Wallentin L, et al: Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med, 2009. 361(11): 1045-57.
- Roe MT, et al: Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med, 2012. 367(14): 1297-1309.
- Montalescot G, et al: Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med, 2014. 371(11): 1016-1027.
- Morice MC, et al: A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med, 2002. 346(23): 1773-1780.
- Moses JW, et al: Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med, 2003. 349(14): 1315-1323.
- Stone GW, et al: A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med, 2004. 350(3): 221-231.
- McFadden EP, et al: Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet, 2004. 364(9444): 1519-1521.
- Lagerqvist B, et al: Stent thrombosis in Sweden: a report from the Swedish Coronary Angiography and Angioplasty Registry. Circ Cardiovasc Interv, 2009. 2(5): 401-408.
- Stone GW, et al: Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med, 2010. 362(18): 1663-1674.
- Planer D, et al: Comparison of everolimus- and paclitaxel-eluting stents in patients with acute and stable coronary syndromes: pooled results from the SPIRIT (A Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (A Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice) Trials. JACC Cardiovasc Interv, 2011. 4(10): 1104-1115.
- Jeger RV, et al: Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet, 2018. 392(10150): 849-856.
- Gwon HC, et al: Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation, 2012. 125(3): 505-513.
- Valgimigli M, et al: Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation, 2012. 125(16): 2015-2026.
- Costa F, et al: Impact of clinical presentation on ischaemic and bleeding outcomes in patients receiving 6- or 24-month duration of dual-antiplatelet therapy after stent implantation: a pre-specified analysis from the PRODIGY (Prolonging Dual-Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia) trial. Eur Heart J, 2015. 36(20): 1242-1251.
- Bittl JA, et al: Duration of Dual Antiplatelet Therapy: A Systematic Review for the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2016. 68(10): 1116-1139.
- Valgimigli M, et al: [2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS.]. Cardiol Pol, 2017. 75(12): 1217-1299.
- Costa F, et al: Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet, 2017. 389(10073): 1025-1034.
- Kim BK, et al: A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy followin Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol, 2012. 60(15): 1340-1348.
- Feres F, et al: Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA, 2013. 310(23): 2510-2522.
- Urban P, et al: Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med, 2015. 373(21): 2038-47.
- Valgimigli M, et al: Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol, 2015. 65(8): 805-815.
- Bonaca MP, Braunwald E, Sabatine MS: Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N Engl J Med, 2015. 373(13): 1274-1275.
- Dewilde WJ, et al: Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet, 2013. 381(9872): 1107-1115.
- Sarafoff N, et al: Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug-eluting stent implantation and an indication for oral anticoagulation. J Am Coll Cardiol, 2013. 61(20): 2060-2066.
- Roldan V, et al: The HAS-BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2-VASc scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol, 2013. 62(23): 2199-2204.
- Gibson, C.M, et al: Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med, 2016. 375(25): 2423-2434.
- Fiedler KA, et al: Duration of Triple Therapy in Patients Requiring Oral Anticoagulation After Drug-Eluting Stent Implantation: The ISAR-TRIPLE Trial. J Am Coll Cardiol, 2015. 65(16): 1619-1629.
- Lamberts M, et al: Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation, 2014. 129(15): 1577-1585.
- Valgimigli M, et al: 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. European Heart Journal, 2018; 39: 213-254.
CARDIOVASC 2019; 18(4): 16-18