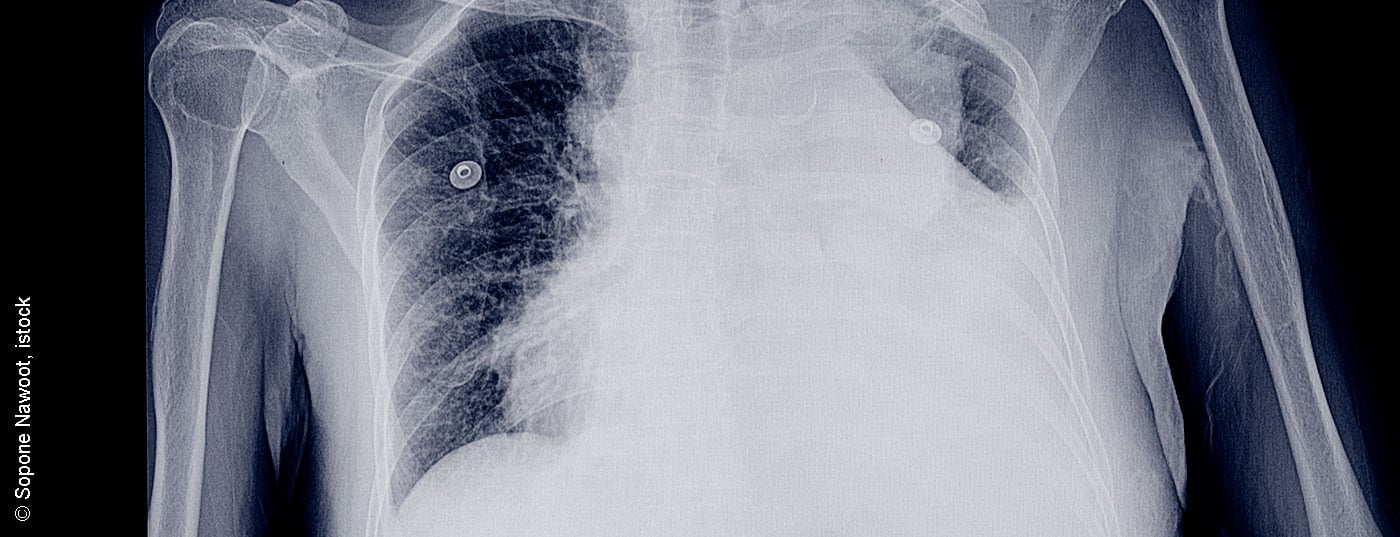
Pleural effusions are common conditions and in many cases are concomitant symptoms of a variety of diseases. An exact diagnosis is crucial for further classification and also for therapy. Some important studies in recent years have been incorporated into the international recommendations.
As the serous skin over the lungs, the pleura is an important part in the respiratory mechanics of the overall system. A thin layer of fluid is necessary to ensure easy sliding of the pleural sheets on top of each other and thus of the lung within the thorax. This allows rapid transmission of force from the respiratory muscles and thoracic excursion to the lungs. The lung itself is coupled to the thoracic wall by this fluid. Pleural mesothelial cells have a passive role in respiratory system lubrification on the one hand, but an active role in the formation of macromolecules, inflammatory cytokines, growth factors, and chemotactic peptides on the other. An important function is the active transport of proteins, lymphatic fluid and cells through the serosa, as well as electrolyte-coupled fluid absorption.
Alteration of pleural permeability due to pathological processes
Inflammation of the pleura and/or adjacent lung structures results in protein leakage into the pleural space due to changes in mesothelial cells and pleural surface structure. The driving forces for increased fluid accumulation are increased hydrostatic pressure, decreased oncotic pressure, and altered lymphatic fluid drainage.
Pleural permeability changes by contact with bacteria, lipopolysaccharides, thrombin [1]. This contact increases the secretion of VEGF in mesothelial cells, which is a potent mediator of permeability increase [2].
Clinic and presentation
Typical symptoms of pleural effusion include chest pain, cough, and dyspnea. Pleuritic pain originates at the parietal pleura because neither the visceral pleura nor the lungs have pain receptors. Typically, it is rapid onset, severe pain with radiation to the shoulder and upper abdomen. As the pleural effusion increases, the pain usually subsides. The cough in pleural disease is a nonspecific symptom, rather dry and persistent, and associated with dyspnea. Dyspnea results initially from fever and pain and with rapidly increasing effusions due to compression of the lungs. Typically, dyspnea is position-dependent [3].
Imaging diagnostics
Pleural effusion and pleural disease can be detected and further clarified by all radiological methods. Posterior-anterior chest radiographs can show effusions greater than 200 m98l, although larger effusions can often be missed on chest radiographs. CT can already detect significantly smaller amounts of effusion and at the same time succeeds in diagnosing a variety of concomitant pathology of the lung and pleura. Underlying diseases such as pneumonia, pulmonary emboli, thoracic malignancies, interstitial lung disease, pleural disease, as well as cardiac and adjacent upper abdominal pathologies can be adequately diagnosed. Differentiation of benign overlays and malignant structures is successful with a sensitivity of 90% [4].
A CT scan should be performed with contrast enhancement and a small amount of residual fluid. Large-volume effusions must be drained first [4]. Thoracic and pleural ultrasound is a safe and innocuous method to diagnose and quantify effusions and to visualize concomitant diseases. A distinction can also be made between simple and complicated parapneumonic effusions, malignant overgrowth, and infiltrates of the lungs. MRI should not be performed routinely. The only diagnostic improvement is in the staging of proven pleural mesothelioma [5].
Invasive diagnostics
Pleural puncture (thoracocentesis) is necessary for differential diagnosis in all pleural effusions after ruling out the major causes of transudate [6]. Transsudative effusions should not be punctured if the cause is clear. In this case, drug therapy of the cause is required first. An ultrasound examination prior to puncture is useful because the puncture site and the amount of punctate can be estimated. Pleural puncture is a very safe invasive method for diagnosis. Pneumothorax occurs in approximately 3-10%. Chemical examination of the pleural fluid usually allows the cause to be explained. Cytology leads to a definite diagnosis in 60% of cases of tuberculosis and in only 25% of cases of malignancy. Negative cytology does not rule out malignant pleural effusion.
There are various pleural blind biopsy techniques for obtaining histological specimens from the pleura. Various needles have been developed for biopsy of the pariental pleura, allowing tissue to be removed for histological examination. The risks here are major bleeding, pneumothorax, and injury to the diaphragm and lung surface. The diagnostic yield is approximately 80% for tuberculosis, 43% for pleural carcinomatosis, and 25% for malignant pleural mesothelioma [7,8].
Medical thoracoscopy as a simple minimally invasive procedure experienced a renaissance with the development of small optical systems and semiflexible endoscopes. Here, optically controlled biopsy of the pleura and lung is possible with a diagnostic sensitivity and specificity of up to 98%.
Surgical video-assisted thoracoscopy (VATS) is an important procedure not only for obtaining biopsies from the pleura and larger wedge resections from the lung but also for various therapeutic procedures such as pleural abrasion, pleurectomy, decortication, and lob and pneumectomies.
Distinction between exudate and transudate
In macroscopically clear effusions, a distinction is made between pure transudates and exudates. Chemical analysis of the punctate and Light’s criteria (measurement of protein and LDH levels in pleural fluid and serum) have the highest sensitivity.
A clinically important criterion is whether effusions occur unilaterally or bilaterally; this helps in the differential diagnosis. Transsudates are typically bilateral, although they can also be unilateral in approximately 20% of cases. Inflammatory effusions usually occur on the side of pathology, with the major exception of systemic disease (as in collagenoses or metastatic carcinoma).
The transsudative pleural effusion
The cause of transsudative effusions is altered pressure conditions in the pleura. An increase in fluid inflow into the pleura on the one hand and a decrease in fluid outflow on the other hand occur with increased venous pressure in the systemic circulation (right heart failure), in the pulmonary circulation (left heart failure), in the portal circulation (liver cirrhosis) or with decreased pleural pressure in pulmonary atelectasis or pulmonary embolism. The amount of fluid depends on the changes in pressure and filtration through the pleura. The most important causes are left heart failure and kidney disease with protein loss.
The exudate in the pleura
The basis for any exudate is a pleural inflammatory response. Inflammation of the pleura can result either from infection or from involvement of the pleura in a variety of systemic diseases. Infectious pleurisy is common with pleural involvement in the setting of pneumonia. This can range from simple concomitant pleurisy to simple and complicated parapneumonic effusions to pleural empyema. The degree and type of inflammatory reaction can be seen by the number and type of inflammatory cells in the pleural fluid.
After chemistry to distinguish transudates from exudates, cytologic workup of the effusion is next of critical diagnostic importance. Neutrophil granulocytes are found in early bacterial infections up to pleural empyema. Lymphocytic effusions are typical in tuberculoses, sarcoidoses, lymphomas and collagenoses, and all malignancies, whereas we find eosinophilic effusions in pulmonary emboli, asbestoses, aspergilloses, mesotheliomas, and pleural carcinoses. “Old” effusions show an increased population of lymphocytes in the punctate [4].
The more inflammatory cells present in the pleura, the more likely it is that there will be congestion, fibrin formation, membrane formation, and adhesions. In parapneumonic effusions, pleural empyema may subsequently develop. Here, bacteriological culture is usually negative because anaerobes are often involved, which are difficult to culture. For parapneumonic effusions, the graduation is as follows:
- Uncomplicated parapneumonic effusion: exudate, predominantly neutrophilic
- Complicated parapneumonic effusion: increasing bacterial invasion: pH decreases, glucose decreases, LDH increases. Fibrin deposition and membrane formation occur.
- Empyema = pus in the punctate. Surgical intervention is usually necessary here.
Common causes of exudates are malignant effusions, which are most common in bronchus, breast, or ovarian carcinomas (Table 1).
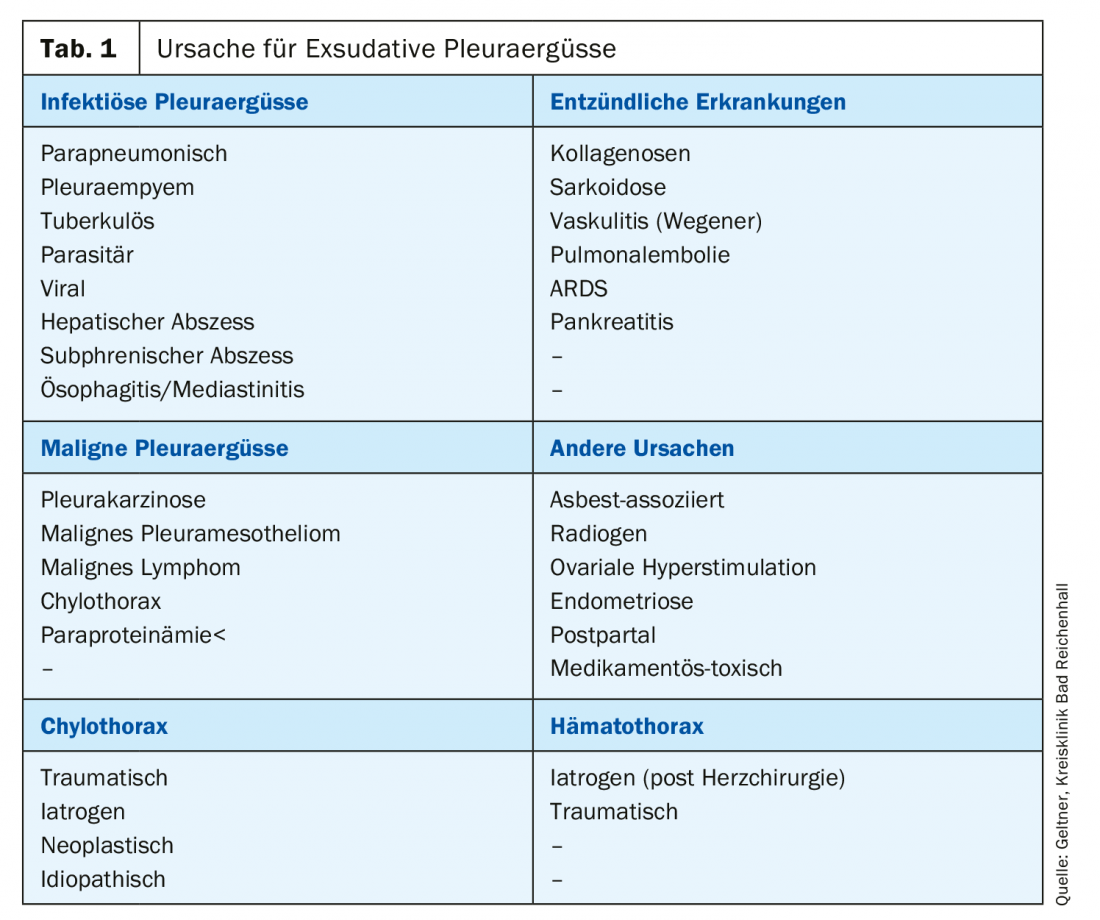
Management of pleural effusion
The first step in the management of an effusion is diagnostic workup. The British Medical Society has developed a practical algorhythm in its guidelines (Fig. 1).

Management of malignant effusion
Malignant effusions and pleural carcinomatosis represent a common complication of thoracic and extrathoracic malignancies [9]. They are very often clearly symptomatic and require palliative therapy (Fig. 2). Curative therapy is usually no longer possible at this stage [10].
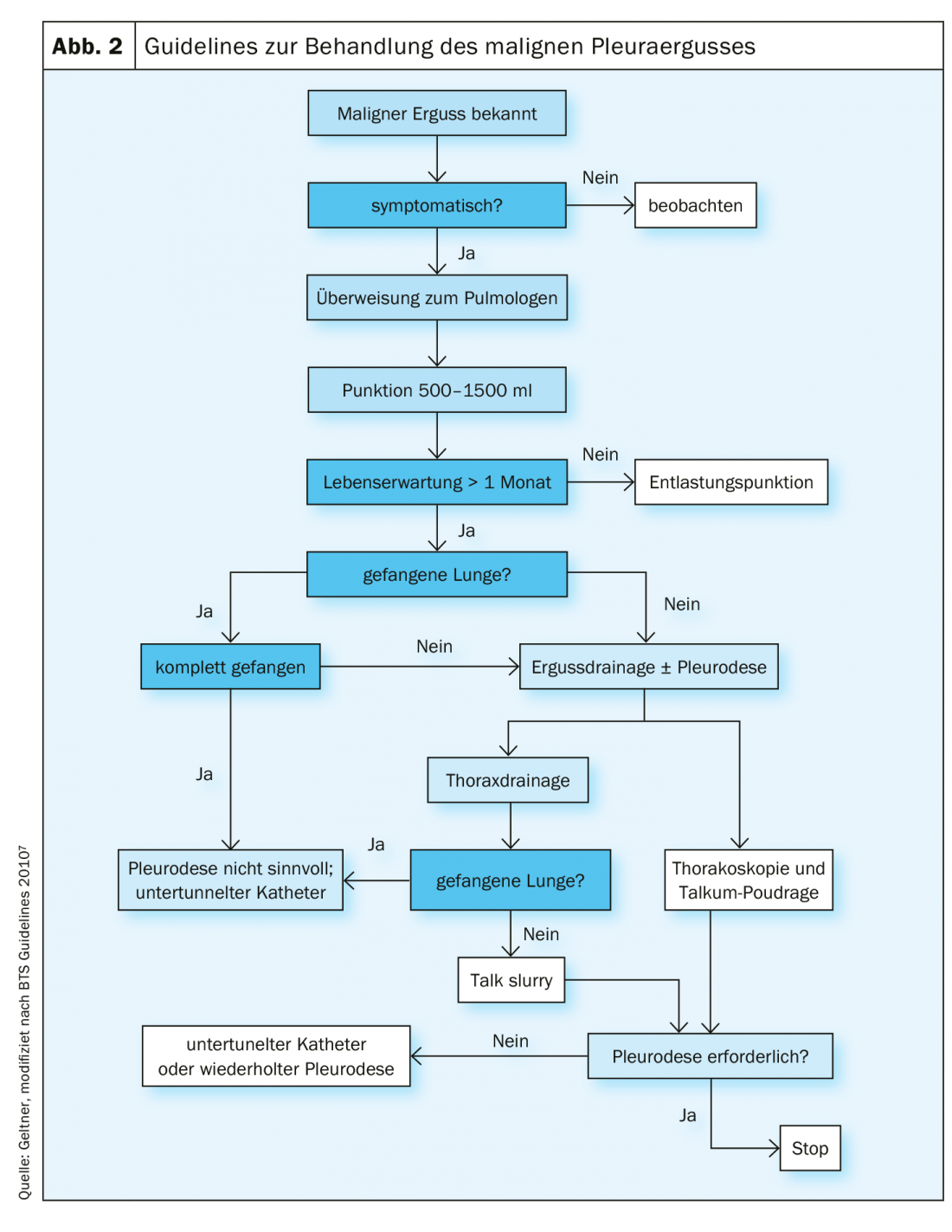
Relief puncture is the method of first choice in symptomatic patients. Repeated punctures should not be performed in patients with a life expectancy of more than one month because of the risk of pleural expectancy and the formation of a “trapped” lung. In this case, it is recommended to perform pleurodesis. Discharge of more than 1.5 liters poses a risk of re-expansion edema of the lungs. In such a case, slow drainage or placement of a chest drain is preferred. Relief puncture has a high recurrence rate of effusion and a low risk of pneumothorax or empyema [11].
For patients with an acceptable general condition and a life expectancy of more than one month, chest drainage followed by pleurodesis should be performed. Sclerosing agents produce sterile inflammation of the pleura and increase fibrinolytic activity. Administration of steroids reduces the effect of pleurodesis. Talc is the most effective and thus recommended substance for pleurodesis.
Non-steroidal anti-inflammatory drugs (NSAID) are generally possible after pleurodesis. Pain management is the same as with opiates (but not better). There is no proven negative effect on pleurodesis rate, so NSAID after pleurodesis is quite reasonable and also recommended (TIME-1-RCT) [12].
The diameter of recommended chest drains has continued to decrease. Due to less pain, thin drains (12F) were recommended by the 2010 guidelines. However, these drainage systems, which are commonly used for pleural effusions, show no advantage in terms of pain sensation and have poorer results in terms of the success of talc pleurodesis.
Permanent implantable pleural catheters (IPC) [13] (Fig. 3) : IPCs are easy to implant and very safe in terms of infection and complication rates. Patients will be able to perform unloading punctures via simple vacuum unloading systems in self-management. If necessary, pleurodesis can also be performed via these catheters. Implantation is usually performed on an outpatient basis.

The combination of IPCs over which talc was then applied increased the acute pleurodesis rate twofold. Complete pleurodesis is achieved in 45% of cases after 5 weeks. From today’s point of view, talc application should be performed after an IPC has been created. Removal is thus possible earlier in numerous cases [14].
Minimally invasive medical thoracoscopy is the method of choice for performing pleurodesis [15]. Under local anesthesia with sedoanalgesia, thoracoscopy can be performed in most patients. No anesthesia with double lumen intubation is necessary. Even in very advanced disease and poor pulmonary or cardiac function, sufficient pleurodesis can be achieved via talcum poudrage The success rate is between 80 and 90%.
Summary
The diagnosis of pleural effusion is very complex because a variety of medical conditions can cause pleural effusion. The most important distinction is made between transudative and exudative effusions and their causes. Laboratory chemical parameters and cytological examinations are helpful here. Depending on the cause, further therapy can be initiated. Numerous methods are available for this purpose, ranging from simple decompression puncture to pleural drainage and thoracoscopy.
Literature:
- Krogel C: 1997. Immunobiology of pleural inflammation: potential implication for pathogenesis, diagnosis and therapy. ERJ 10: 2411-2418.
- Hott JW: 1999. Role of VEGF in the development of malignant pleural effusions. AJRCCM 159: A212.
- Yernault JC: 2002. History, symptoms and clinical examination in pleural diseases. Eur Resp Monograph 22: 60-75.
- Traill ZC, Davies RJO, Gleeson FV: 2001. Thoracic computed tomography in patients with suspected malignant pleural effusions. Clin Radiol 56: 193-196.
- Giesel FL: 2006. Dynamic contrast enhanced MRI of malignant pleural mesothelioma: a feasibility study of non-invasive assessment, therapeutic follow-up, and possible predictor of improved outcome. Chest 129; 1570-1576.
- Hooper C: 2010. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. BMJ, Thorax 65 (Suppl 2): ii4-7.
- Loddenkemper R: 1983. Prospective evaluation of bopsy methods in the iagnosis of malignant pleural effusion. Intrapatient comparison between pleural fluid cytology, blind needle biopsy, and thoracoscopy. ARRD 127: 114.
- Astoul P: 2011. Pleurodesis for recurrent malignant pleural effusions: the quest for the Holy Grail. Eur J Cardiothorac Surg. 40(2): 277-279.
- Geltner C, Errhalt P: 2016. Malignant pleural effusion: pathogenesis, diagnosis, and management. MEMO DOI 10.1007/s12254-015-0246-0; online Nov. 19, 2016.
- Feller-Kopman DJ: 2018. Management of malignant pleural effusion, ATS Guideline. Am J Respir Crit Care Med Vol 198, Iss 7: 839-849.
- Sorensen PG, Svendsen TL, Enk B: 1984. Treatment of malignant pleural effusion with drainage, with and without instillation of talc. Eur J Respir Dis 65: 131-135.
- Rahman NM: 2015. Effect of Opioids vs NSAIDs and Larger vs Smaller Chest Tube Size on Pain Control and Pleurodesis Efficacy Among Patients With Malignant Pleural Effusion: The TIME1 Randomized Clinical Trial. JAMA 314(24): 2641-2653.
- Musani AI: 2009. Treatment options for malignant pleural effusion. Curr Opin Pulm Med 15: 380-387.
- Bhatnagar R: 2018. Outpatient Talc Administration by Indwelling Pleural Catheter for Malignant Effusion. NEJM 378(14): 1313-1322.
- Tschopp JM: 2009. Pleurodesis by talc poudrage under simple medical thoracoscopy: an international opinion. Thorax 64(3): 273-274.
InFo PNEUMOLOGY & ALLERGOLOGY 2019; 1(2): 24-27.