When excess potassium becomes severe, life-threatening cardiac arrhythmias can result. In particular, renal insufficiency, cardiac insufficiency and diabetes, as well as certain medications, favor the development of hyperkalemia. Treatment with oral potassium binders allows potassium levels to be lowered in parallel with the continuation of central therapies such as RAAS inhibitors or aldosterone antagonists. In addition to patiromer, which was approved several years ago, another therapeutic option, sodium zirconium cyclosilicate, has been available in Switzerland since 2021.
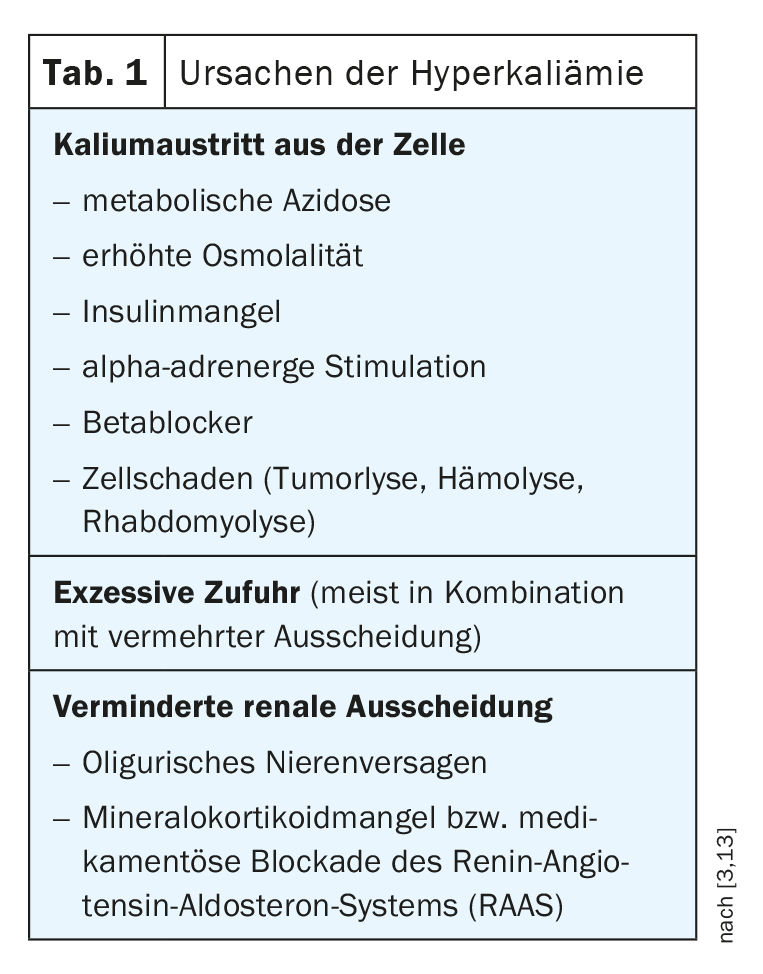
Three processes favor the development of hyperkalemia: increased potassium uptake, increased release of potassium from cells, and decreased elimination (Table 1). Potassium is predominantly localized intracellularly, only about 2% of total body potassium is located in the extracellular space. Blood plasma usually has potassium concentrations between 3.5 mmol/l and 5.0 mmol/l. The gradient between intracellular and extracellular potassium is important for maintaining membrane potential and for excitation conduction of nerve and muscle cells [1]. According to the classical definition, a serum potassium level of >5 mmol/l is considered hyperkalemia [3]. Excess potassium is usually detected during routine blood tests or when certain changes occur in the electrocardiogram. Appropriate monitoring is recommended for patients at risk (box).
Renal insufficiency patients often have elevated serum potassium levels
With an incidence of 2-3% in the general population, hyperkalemia is one of the most common electrolyte disorders in clinical practice [3]. In patients with chronic kidney disease, the incidence is as high as 40-50% [4]. Possible symptoms of hyperkalemia range from muscle weakness and paralysis to cardiac arrhythmias. Severe hyperkalemia is potentially life-threatening. Most commonly, elevated serum potassium levels occur in the context of impaired renal function; in addition, there are several other possible causes. Where usually there is a combination of a clinical risk factor with one or more potassium-increasing drugs (eg, renin-angiotensin-aldosterone (RAAS) inhibitors or aldosterone antagonists) [2,3] (box) .
|
Check potassium levels regularly in patients at risk! Especially in the treatment of cardiac or renal insufficiency, hyperkalemia is a common problem. Hyperkalemia is often the result of decreased renal excretion due to acute or chronic renal insufficiency. Drugs that affect the renin-angiotensin-aldosterone system (RAAS) are another common cause, as these are among the serum potassium elevating agents [10]. Therefore, regular monitoring of potassium levels is recommended in patients receiving RAAS inhibitors or aldosterone antagonists. If hyperkalemia occurs during the course of therapy, appropriate countermeasures are required. Life-threatening ECG changes with arrhythmias and even cardiac failure may occur, particularly if serum potassium levels exceed 7 mmol/l. In these cases, inpatient treatment is required. |
When are oral potassium binders indicated?
Some well-performing agents are available for acute or subacute hyperkalemia (e.g., calcium gluconate, insulin plus glucose, beta-adrenoreceptor agonists, loop diuretics, sodium bicarbonate). But for maintenance or long-term control, only a change to a low-potassium diet, dose reduction or discontinuation of potassium-lowering drugs could be recommended so far. However, this is not always possible. Oral potassium binders were launched to close this therapeutic gap. Patiromer (Veltassa®) has been on the market in Switzerland since 2017 and sodium zirconium cyclosilicate (Lokelma®) received Swissmedic approval in April 2021. Both are oral potassium binders with evidence-based efficacy that can be used for moderate hyperkalemia. However, if acute severe hyperkalemia is present, inpatient treatment is required – according to the classical classification, hyperkalemia is considered severe when serum potassium is ≥6.5 mmol/l.
Potassium-boosting drugs do not have to be discontinued
Sodium zirconium cyclosilicate binds potassium in a crystalline lattice structure in exchange for sodium and hydrogen and is not absorbable [6]. In a double-blinded study, 753 patients with chronic renal failure and hyperkalemia of various etiologies received sodium zirconium cyclosilicate at a dose of 1.25 g, 2.5 g, 5 g, or 10 g three times daily for 2 days or placebo [7,8]. Patients in whom potassium levels normalized were subsequently treated with sodium zirconium cyclosilicate or placebo once daily from day 3 to day 14. The primary endpoint was potassium change (“mean exponential rate of changes”) at 48 hours. 75% of patients had chronic renal failure stage III or worse (GFR <60 ml/min), 60% were diabetic, and 40% suffered from heart failure. 65% of subjects were on treatment with RAS blockade during the study. Comorbidities were well balanced at randomization, with those patients receiving sodium zirconium cyclosilicate or placebo at 10 g having slightly higher potassium levels. After 48 hours, the treatment arms in the 2.5 g, 5 g, and 10 g dosing groups showed a decrease in serum potassium from 5.4 mmol/l (baseline) to 4.9 mmol/l and 4.8 mmol/l and 4.6 mmol/l, respectively. In the placebo and 1.25-g groups, the value decreased only to 5.1 mmol/l. In the subsequent study phase, serum potassium levels of 4.7 and 4.5 mmol/l were maintained in the verum groups using 5 g and 10 g sodium zirconium cyclosilicate, respectively, and leveled off at 5.0 mmol/l in the placebo group. Side effects occurred only rarely.
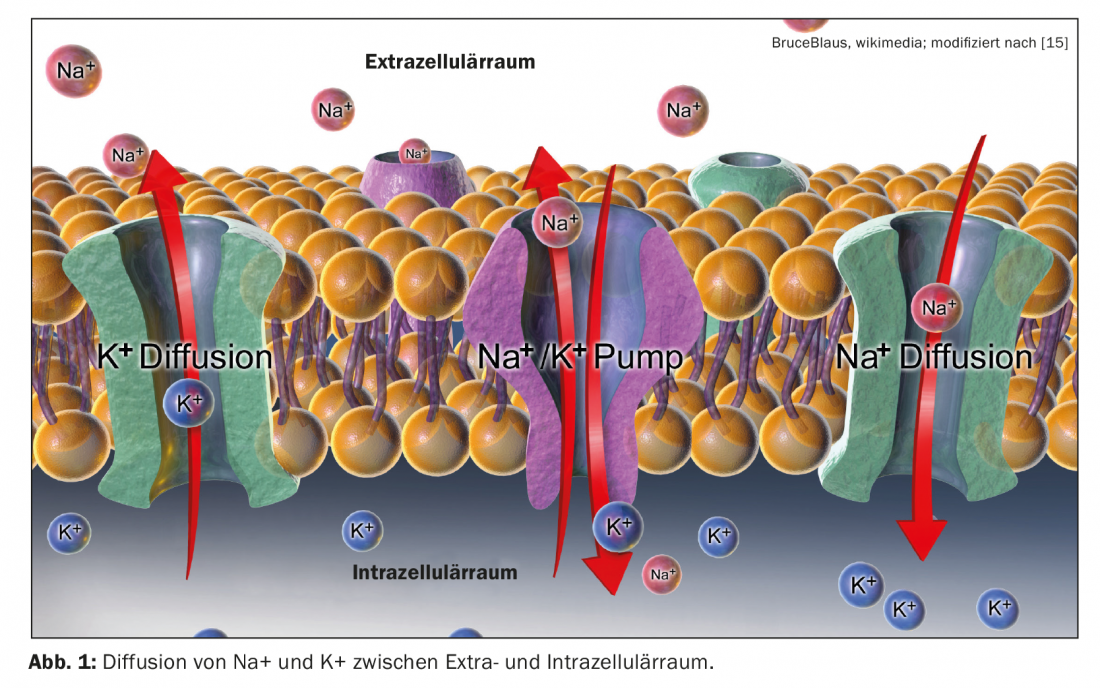
Patiromer [12] is a non-absorbable polymer that binds potassium in the colon and exchanges it for calcium. In a study by Weir et al. 237 patients with chronic renal failure (GFR 15-59 ml/min) taking renin-angiotensin system (RAS) inhibitors with potassium between 5.1 and 6.5 mmol/l were treated in a single-blind fashion with 4.2-8.4 g patiromer twice daily for four weeks [7,9]. 76% of study participants achieved normal potassium levels with an average potassium reduction of 1 mmol/l for all patients with the main effect in the first few days. Subsequently, 107 patients of the study population were included in a placebo-controlled discontinuation phase of eight weeks. Hyperkalemia recurred in 60% of patients in whom patiromer was discontinued, with the potassium rebound manifesting particularly in the first two weeks. Among those who continued to receive Patiromer, this proportion was only 15%. The most common adverse event was constipation (11%), and hypokalemia occurred in 3% of all study participants.
With regard to long-term therapeutic effects, the results of the AMETHYST-DN study are interesting. This demonstrated that after four weeks of treatment with Patiromer, potassium levels remained within the target range of 3.8-5.0 mmol/l over a 52-week period.
Literature:
- Giant WF: Electrolyte disorders – frequently seen, often underestimated. Pathological concentrations of sodium, potassium and calcium. the informed physician 2013(8): 28-31.
- Swiss Ultrasound Center and Institute of Rheumatology, www.irheuma.com/de/index.php?p=rheumatology-a-z/a-1-3-1/electrolyte-disorder (last accessed 07.01.22).
- Zieschang S: Hyperkalemia in everyday practice, Arzneimittelkommission der deutschen Ärzteschaft, www.akdae.de/Arzneimitteltherapie/AVP/Artikel/201901-2/059h/index.php, (last accessed 07.01.22).
- Kovesdy CP: Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 2014; 10: 653-662.
- Montford JR, Linas S: How Dangerous Is Hyperkalemia?J Am Soc Nephrol 2017; 28: 3155-3165.
- Swissmedic: Lokelma®, www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/authorisations/new-medicines/lokelmatm-pulver-fuer-suspension.html (last accessed 07.01.22).
- Weir MR, et al: OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372(3): 211-221.
- Packham DK, et al: Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372: 222-231.
- Hallwachs A: Pharmaceuticals – a critical look. Does the country need new potassium-lowering agents? Arzneimittelkommission der deutschen Ärzteschaft, www.akdae.de/Arzneimitteltherapie/AVP/Artikel/201504/174h/index.php, (last accessed 07.01.22).
- ifap Service-Institut für Ärzte und Apotheker GmbH, www.ifap.de/deu_de/magazin/artikel-neueinfuehrung/neues-arzneimittel-gegen-hyperkaliaemie-bei-erwachsenen.html (last accessed 07.01.22)
- National Health Care Guideline (NVL) Chronic Heart Failure, 3rd edition, www.leitlinien.de/themen/herzinsuffizienz# (last accessed 07.01.22).
- Swissmedic: Veltassa®, www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/authorisations/new-medicines/veltassa_pulver_fuer_orale_suspension_patiromer.html (last accessed 07.01.22).
- Palmer BF, Clegg DJ: Diagnosis and treatment of hyperkalemia. Cleve Clin J Med 2017; 84: 934-942.
- Bakris GL, et al; AMETHYST-DN Investigators. Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA 2015;314(2): 151-161.
- WikiJournal of Medicine: Medical gallery of Blausen Medical 2014, DOI:10.15347/wjm/2014.010.
HAUSARZT PRAXIS 2022; 17(1): 36-37
CARDIOVASC 2022; 21(1): 32-33