Tumor screening with tumor markers alone is not useful, except for PSA/prostate cancer. Screening for prostate cancer must be discussed with the patient on an individual basis. Tumor markers can supplement the standard during the initial diagnosis of a tumor, during therapy monitoring, and during follow-up.
Serum tumor markers or systemic tumor markers (hereafter referred to as tumor markers) are formed and secreted in malignant tumor cells. Tumor markers can be detected in peripheral blood. The commonly used biochemical markers are tumor-specific molecules such as enzymes, hormones, growth factors or metabolites. Based on their specificity and sensitivity, tumor markers are used for screening, diagnosis, tumor therapy monitoring or in follow-up. Certain tumor markers have thus gained a firm place in clinical routine, but the indications refer to a relatively small group of tumor patients. The authors of this article are of the opinion that currently too many tumor marker determinations are performed, which in certain cases can also lead to anxiety and uncertainty among patients. This article is intended to provide a brief overview of the recommended use of tumor markers.
Tumor markers in germ cell tumors
Germ cell tumors (CSCT) include teratoma, dysgerminoma, yolk sac tumors, and chorionic carcinoma in females, and seminoma and non-seminomatous CSCT (yolk sac tumor, chorionic carcinoma, embryonal carcinoma, teratoma) in males. In these tumors, alphafetoprotein (AFP), β-human chorionic gonadotropin (β-HCG), and lactate dehydrogenase (LDH) have significance as tumor markers.
Alphafetoprotein (AFP)
AFP is produced in the fetal yolk sac, fetal liver, and fetal intestine. The mirror reaches its maximum level during the 12th-14th week of the year. The peak value is reached in the first week of pregnancy and then falls linearly. By one year of age, AFP has dropped to low levels. AFP can be detected in embryonal carcinoma or teratoma (20-25%), non-seminomatous CCT (60%) and hepatocellular carcinoma (41-65%) [1]. Pure chorionic carcinomas and pure seminomas do not produce AFP. When AFP is elevated in seminomas and pure chorionic carcinomas, this is an important indication of mixed tumors.
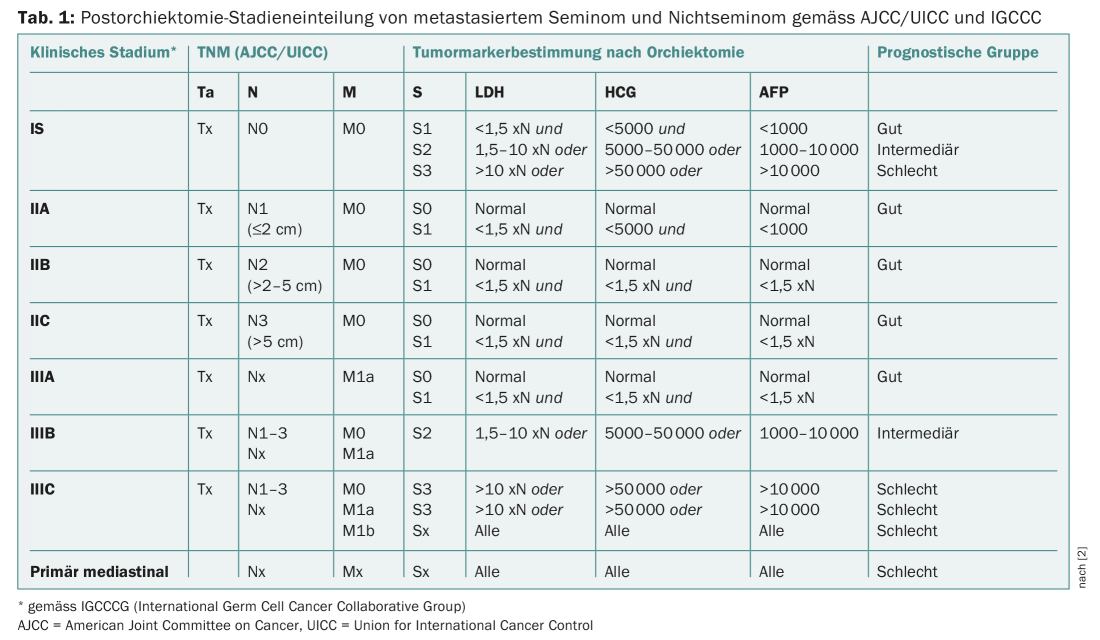
APF, β-HCG and LDH are part of the diagnostic and prognostic classification of CCT (Tab. 1) . False positive values can occur in hepatopathies (cirrhosis, hepatitis), in drug and alcohol abuse, and rarely as a familial idiopathic form. The half-life of AFP is five to seven days. After effective treatment, AFP levels should normalize according to these kinetics. A high AFP level (>10,000 ng/ml) is associated with poor prognosis in seminoma and non-seminomatous CCT [2]. AFP and β-HCG also have diagnostic value in tumor follow-up.
β-human chorionic gonadotropin (β-HCG)
β-HCG is markedly elevated in non-seminomas or chorionic carcinomas and only moderately elevated in embryonal cell carcinomas or mixed CCT. β-HCG elevations are also seen in bladder mole and placental trophoblastic tumors. The serum half-life of β-HCG is 24-36 hours. Plasma concentrations of β-HCG normalize after orchiectomy according to these kinetics or persist in residual tumor disease [3].
False positive levels of β-HCG occur in hypogonadism, tumor lysis syndrome, heterophilic antibodies, use of marijuana, and pregnancy. In stage I seminomas, β-HCG is elevated in 10-20% of patients, and in disseminated seminomas (secondary to the presence of syncytial trophoblast fractions) in 30-50% of patients (rarely >500 mIU/ml). In bladder mole, systemic β-HCG levels typically exceed 100,000 mIU/ml and are even higher than in pregnancy. Placental trophoblastic tumors show low β-HCG elevation, whereas epithelioid trophoblastic tumors show low or even absent β-HCG expression.
Lactate dehydrogenase (LDH)
LDH is a low sensitivity and low specificity tumor marker in patients with non-seminomatous CCT. LDH is elevated in 40-60% of men with testicular CCT. The degree of LDH elevation has prognostic value in men with advanced CCT.
Clinical application of AFP, β-HCG and LDH
Serum levels of AFP, β-HCG and LDH are determined for correct staging at initial diagnosis of CCT. Additional determination of these tumor markers is performed after orchiectomy, followed by weekly controls until complete normalization of the initially elevated tumor markers. After orchiectomy, the three tumor markers are used for risk stratification (Table 1). Men with non-seminomatous CCT and persistently elevated AFP or β-HCG after orchiectomy must be assumed to have metastatic tumor stage. After primary therapy is completed, tumor markers (β-HCG, AFP, and LDH) are used as a supplement to radiological follow-up. Depending on histology, stage, and prior therapy, the algorithm calls for three-monthly checks until the third year, followed by six-monthly checks until the fifth year, and annual checks until the tenth year [4]. In an older retrospective study, 125 patients with recurrent CCT were studied. Among these, tumor marker elevation was the earliest indicator of recurrence in nonseminomas (35/87 patients = 40%). In seminoma, imaging was the earliest indicator of recurrence (9/36 patients = 42%) [5].
PSA as a tumor marker in prostate cancer
PSA (prostate-specific antigen) is produced in the glandular cells of the prostate as a proenzyme (proPSA). The proPSA is converted into the active PSA by proteolysis. Active PSA is either proteolyzed and passes into the bloodstream in an unbound form (free PSA), or it is rapidly bound as unproteolyzed PSA to protease inhibitors such as α1-antichymotrypsin or α2-macroglobulin. Free and bound PSA are determined together as total PSA. The half-life of PSA is two to three days.
PSA screening in practice
Broad PSA screening is not recommended. Selective PSA screening after detailed patient education may be considered, especially in high-risk patients who have a significantly increased risk of developing biologically relevant prostate cancer:
- Men of African ethnicity
- Men with father or brother suffering from prostate cancer
- Patients with germline mutations of BRCA 1 or BRCA 2.
- Men with Lynch syndrome (hereditary non-polyposis colon cancer).
PSA screening begins with an initial PSA determination at age 50 (45 years for high-risk patients) and is usually combined with a digital-rectal examination. A single elevated PSA value should be repeated. Various influences on the PSA value are significant: physiological increase with higher age, ethnic differences and effect of drugs (increase under anabolic steroids, decrease by up to 50% under α1-reductase inhibitors). Asymptomatic men older than 70 years should not receive PSA screening [6].
As a guideline, for prostate cancer screening and a PSA <1 ng/ml, a next control can be done after three years, for PSA from 1 to <2 ng/ml after two years, and for PSA from 2 to <3 ng/ml after one year [7]. False high PSA levels may also occur in patients with benign prostatic hyperplasia and inflammatory or infectious prostatitis. Digital-rectal palpation or prolonged cycling can also cause PSA elevation, so PSA levels should be determined beforehand. After ejaculation, the PSA level may be elevated by 0.4-0.5 ng/ml for two to three days.
Alternative proposals for broader PSA screening have recently been published. The long-term prognostic significance of PSA is interesting. For example, it has been shown that 60-year-old men with PSA >2 ng/ml have a significantly increased risk of dying from prostate cancer [8]. Patients with a PSA >100 ng/ml at diagnosis have significantly reduced overall survival. Some men with agressive prostate cancers (neuroendocrine, undifferentiated) have a low PSA as a sign of low tumor cell differentiation. The low sensitivity of the PSA is problematic: it is 20% with a PSA cutoff of 4.0 ng/ml and average disease risk resp. 50% in high-risk patients. This can lead to a significant number of negative biopsies with corresponding patient uncertainty. Biopsies of the prostate also carry the risk of complications such as fever, chills, pain, hematuria, hematochezia (fresh blood in the stool), and bloody ejaculate.
PSA screening studies
The clinical utility of PSA screening has been evaluated in two large randomized trials (ERSPC = European Randomized Study for Screening for Prostate Cancer [9] and PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial [10]), both of which had the endpoint of prostate cancer-specific mortality.
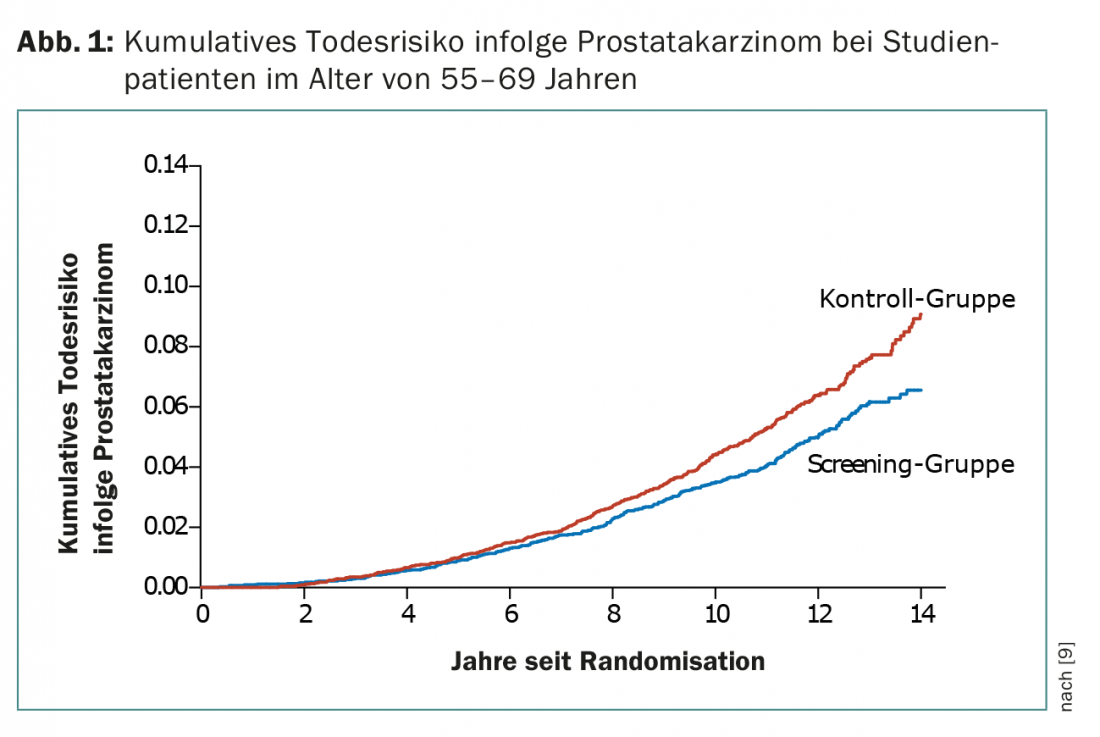
In the ERSPC study, 162 243 men aged 55-69 years were randomized to a group with PSA determination every four years or to the control group without PSA screening. After a median follow-up of nine years, the cumulative incidence of prostate cancer was 8.2% in the screening group and 4.8% in the control group. The ratio of deaths due to prostate cancer was 0.8 (PSA screening group/control group) (Fig. 1) . This results in a 20% lower mortality in the screening group, but with a greater risk of overdiagnosis. Data after eleven years of follow-up showed that to prevent one death, 1055 men must be screened and 37 prostate cancers diagnosed [9].
In the PLCO study, 76,693 men were assigned to either the screening group (annual PSA for six years and annual digital rectal examination for four years) or the control group. No reduction in mortality was shown in the screening group [10]. After seven years of follow-up, the incidence of prostate cancer per 10,000 person-years was 116 (2820 tumors) in the screening group and 95 (2322 tumors) in the control group (RR 1.22). The incidence of deaths per 10,000 person-years was 2.0 (50 deaths) in the screening group and 1.7 (44 deaths) in the control group (ratio 1:1.13) [10].
The assessability of the large PSA screening studies is limited, among other things, by “contamination” (PSA screening in the control arm) and the fact that some of the subjects had already undergone PSA control before study entry. Based on the data presented, unselected PSA screening is not currently recommended.
Further diagnostics for elevated PSA values
PSA values in the range of 2.5-10 ng/ml, which may already represent a pathological value depending on age and prostate volume, are a particular diagnostic challenge. Here, so-called “PSA derivatives” may have an additional diagnostic benefit:
- PSA ratio: ratio of free to total PSA
- PSA density: prostate volume divided by total PSA
- PSA velocity: PSA increase over time.
An annual PSA increase >0.75 ng/ml is suspicious for the presence of prostate cancer. The PSA ratio improves the sensitivity of the diagnosis of prostate cancer when the total PSA level is in a range between 4-10 ng/ml. A low PSA ratio is indicative of the presence of prostate carcinoma [11], although no uniform cut-off was used (10-25%). In cases of abnormal PSA and/or pathologic digital-rectal findings, MRI of the prostate should be considered, which allows for targeted biopsy sampling if pathology is appropriate.
PSA control after prostatectomy
Patients who have undergone radical prostatectomy have biochemical recurrence if the serum PSA level exceeds 0.2 ng/ml twice. Salvage radiotherapy should be given early (PSA <0.5 ng/ml) [12]. Biochemical recurrence after radiotherapy with or without hormonal therapy is present when the PSA level >2 ng/ml rises above the nadir [13,14].
Therapy monitoring by means of PSA
Castration-resistant prostate cancer is present in patients who experience disease progression on androgen deprivation therapy, defined as a sequential PSA rise of ≥2 ng/mL over nadir within at least one week. Here, testosterone must be adequately suppressed, defined as serum testosterone <50 ng/l (1.7 nmol/l). After starting therapy, PSA values should be determined again after twelve weeks at the earliest (caveat “PSA flair”); in case of a PSA increase, this should be confirmed by means of a second PSA determination after ≥3 weeks [15].
Other tumor markers
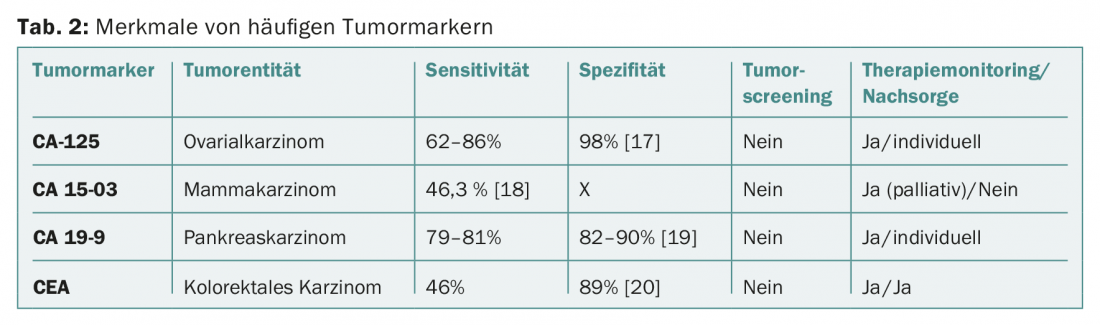
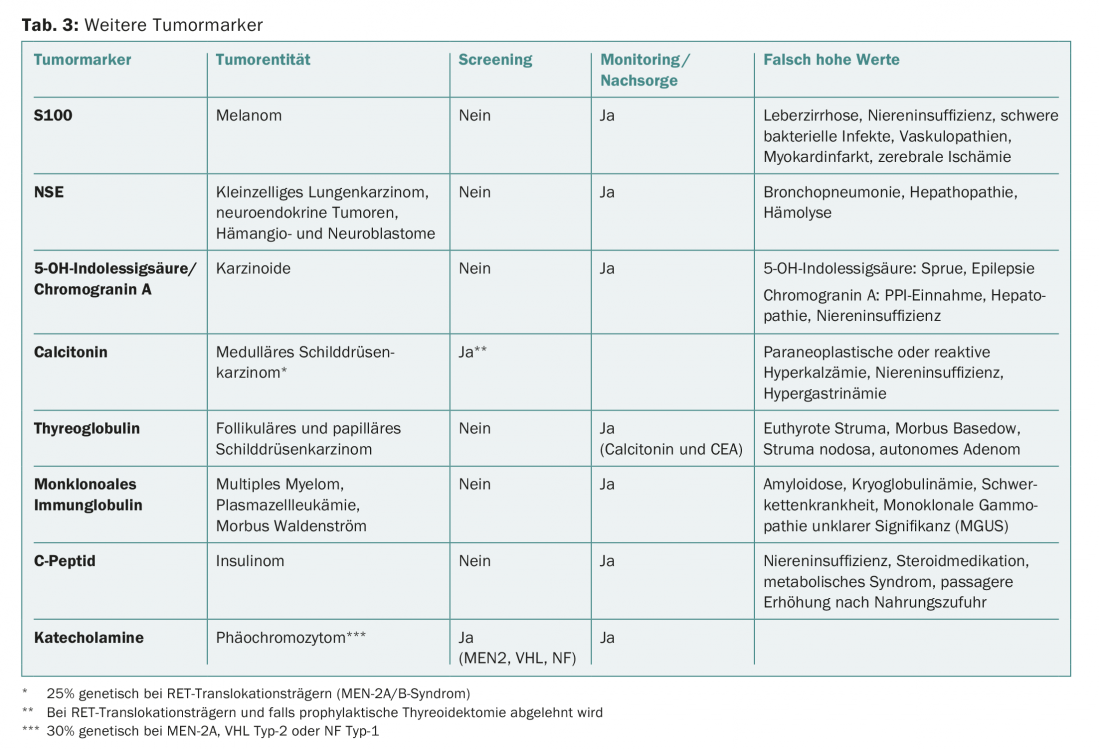
Tumor markers are most commonly used for therapy monitoring. The specificity and sensitivity of most serum tumor markers are too low to be usefully applied for tumor diagnosis or screening (Tab. 2). Exceptions are known familial tumor syndromes such as Von Hippel-Lindau syndrome (VHL) or multiple endocrine neoplasia (MEN) (Tab. 3).
CEA is a relatively unspecific marker, but has a fixed place in tumor follow-up for curatively treated colon or rectal carcinomas (determination every three months in the first year, every six months in the second and third year, annually in the fourth and fifth year).
CA-125 is frequently used in therapy monitoring as well as in the follow-up of ovarian cancer. However, biochemical recurrence with sequentially increasing CA-125 without corresponding clinical symptoms should not be considered an indication for cytotoxic recurrence [16].
Literature:
- Gupta S, et al.: Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C.. A systematic review and critical analysis. Ann Intern Med 2003; 139: 46-50.
- Oldenburg J, et al: Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment, follow-up. Ann Oncol 2013; 24 Suppl 6: vi125-132.
- Bosl GJ, Motzer RJ: Testicular germ-cell cancer. N Engl J Med 1997; 337: 242-253.
- Milose JC, et al: Role of biochemical markers in testicular cancer: diagnosis, staging, and surveillance. Open Access J Urol 2011; 4: 1-8.
- Trigo JM, et al: Tumor markers at the time of recurrence in patients with germ cell tumors. Cancer 2000; 88: 162-168.
- Parker C, et al: Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26 Suppl 5: v69-77.
- Gasser T, et al: PSA determination – Recommendations of the Swiss Society of Urology (SGU). Schweiz Med Forum 2012; 12(6): 126-128.
- Vickers AJ, et al: Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ 2010; 341: c4521.
- Schroder FH, et al: Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384: 2027-2035.
- Andriole GL, et al: Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012; 104: 125-132.
- Catalona WJ, et al: Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 1998; 279: 1542-1547.
- Lilja H, et al: Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer 2011; 117: 1210-1219.
- Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys 1997; 37: 1035-1041.
- Roach M, et al: Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65: 965-974.
- Scher HI, et al: Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148-1159.
- Rustin GJ, et al: Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet 2010; 376: 1155-1163.
- Menon U, et al: Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol 2005; 23: 7919-7926.
- Molina R, et al: C-erbB-2, CEA and CA 15.3 serum levels in the early diagnosis of recurrence of breast cancer patients. Anticancer Res 1999; 19: 2551-2555.
- Ballehaninna UK, Chamberlain RS: The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 2012; 3: 105-119.
- Liu Z, et al: A systematic review and meta-analysis of diagnostic and prognostic serum biomarkers of colorectal cancer. PLoS One 2014; 9: e103910.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(4): 5-9.