Actinic keratoses (AK) are associated with squamous cell carcinoma, but little is known about the risk of progression of individual lesions. However, field carcinogenesis or a higher number of lesions and the extent of hyperproliferation of atypical keratinocytic cells seem to be relevant factors. A good and feasible tool for assessing the severity of AK and evaluating treatment success is the AKASI score. Nowadays, the diagnosis of AK can be confirmed by modern non-invasive methods.
Actinic keratoses (AK) are among the most common dermatoses on chronically sun-damaged skin. These are precancerous skin lesions resulting from proliferation of atypical keratinocytes on UV-damaged skin, particularly affecting the face, hairless scalp, and extremities. According to epidemiological data, the prevalence of AK in people over 60 years of age is between 20-35% [1–3].
AK is a facultative precancerous condition
A cohort study with a follow-up period of 5 years showed that 65% of squamous cell carcinoma (SCC) arose from preexisting AK lesions [4]. In another study, the probability of progression to SCC within 10 years was reported to be about 10% in patients with an average of 7.7 AK [19].
“Genome sequencing studies confirm that actinic keratoses are precancerous lesions,” said Prof. Dr. Nicole Kelleners-Smeets of Maastricht University Medical Center (NL) [5]. The conclusion of a large molecular genetic study using exome sequencing was that AK is characterized by genomic alterations that are also present in SCC, including abnormalities in the TGF-β signal transduction pathway [6]. Also in agreement with other studies, mutations in TP53 and NOTCH were also detected. Early mutational inactivation of NOTCH1 in AK may facilitate progression from AK to SCC [6]. TP53 is a tumor suppressor gene whose gene product (p53 protein) functions as a transcription factor. It is activated by cellular stress, for example UV-induced DNA damage. Although there is increasing evidence on factors that promote progression to SCC, assessing which AK lesions are at high risk and which will regress remains difficult. That treatment of AK can prevent the development of SCC is now considered proven, the speaker emphasized.
| The risk of progression from AK to SCC is estimated to be 0.025% to 16% for a single lesion per year. Accordingly, transformation rates for a patient with 6-8 lesions are 0.15-80% per year [9]. Currently, the evidence base for prognostic factors determining a transition from AK to SCC remains insufficient [9]. Recent studies indicate that the previous staging of AK and estimation of progression risk should be reconsidered [13,14]. In the context of the occurrence of AK in the context of field cancerization and a positive correlation between number of lesions and the probability of progression, new evaluation criteria for the stages of AK are proposed [9]. The AKASI (actinic keratosis area and severity index) is currently a meaningful tool to assess the severity of AK, but also to evaluate the success of treatment [12]. |
Use non-invasive methods for the diagnosis of AK
According to the current S3 guideline, the indication for treatment of AK should be based on the clinical picture and risk factors (e.g., immunosuppression, cumulative UV exposure, number of lesions) [9]. Histologically, one characteristic of AK is the accumulation of atypical keratinocytes in the stratum basale of the epidermis, from where they may progress over time to the stratum granulosum and stratum corneum [16,17]. The altered keratinocytes of AK exhibit enlarged, pleomorphic, hyperchromatic nuclei and high nuclear-cytoplasmic ratio [9]. While histopathologic examinations used to be required to identify precancerous changes in the skin, several noninvasive diagnostic techniques are now available, particularly dermoscopy, confocal reflectance microscopy (RCM), and optical coherence tomography (OCT) or line-field OCT, explained Prof. Giuseppe Micali, M.D., University of Catania (I) [7]. Dermatoscopy has high sensitivity (approximately 98%) and specificity (approximately 95%) for the detection of AK, according to the speaker [7]. However, pigmented AK is sometimes difficult to differentiate clinically and dermoscopically from other skin lesions, such as lentigo maligna. In addition to dermoscopy, RCM is another non-invasive diagnostic technique available. RCM allows visualization of the cytomorphologic substrate of suspicious dermatoscopic structures at the cellular level [8]. The light-damaged skin often shows an atypical honeycomb pattern in a field cancerization as a sign of subclinical AK [9]. A new non-invasive imaging technique is LC-OCT. By imaging individual cells at a high resolution with a penetration depth of up to 500 μm, diagnostic accuracy can be significantly increased.
AKASI as a tool for assessing the severity level
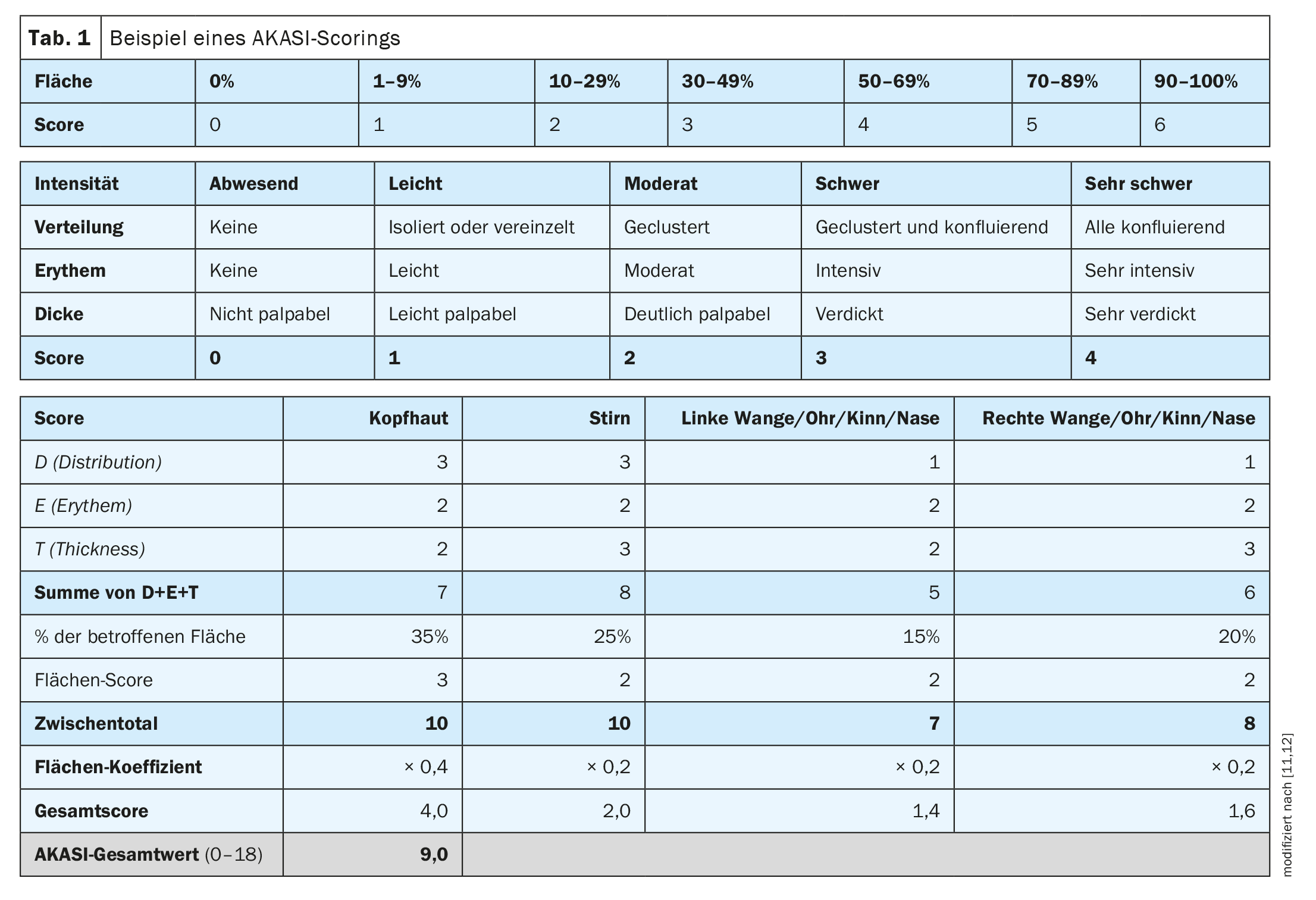
In clinical practice, it is also a matter of assessing the severity of actinic keratoses as a basis for treatment decisions, said Dr. Girish Gupta, dermatologist and Senior Clinical Lecturer, Edinburgh University (UK) [10]. “We know that squamous cell carcinoma is associated with AK,” the speaker explained [10]. The AKASI (“Actinic keratoses activity and severity index”) was developed to assess and monitor severity [12]. “Maybe we can use the AKASI score to assess risk,” Dr. Gupta said. That the AKASI is a very good tool to objectively assess the severity of AK is also in line with the opinion of Prof. Dr. Thomas Dirschka, Centroderm Klinik Wuppertal (D) [11,12]. The AKASI allows for easy quantification of lesions (Table 1) [11,12]. In a 2018 study by Schmitz et al. was investigated whether and how the AKASI score is associated with keratinocytic tumors [18]. It was concluded that patients with an AKASI screen >7 are likely to be at higher risk of developing invasive SCC compared with AK patients with a lower score.
Regarding the non-invasive methods described, Prof. Dirschka highlighted LC-OCT as a very good diagnostic method to detect potentially dangerous lesions. Both horizontal and vertical sections as well as histological aspects are displayed in real time.
Risk for malignant transformation correlates with the number of lesions
“The most important goal is to prevent progression to SCC,” Prof. Dirschka said, adding, “We need therapies for high-risk patients.” [11]. The higher the number of lesions, the higher the risk that one of the lesions will develop into SCC. Therefore, the number of lesions and field cancerization play an important role in progression risk, the speaker said. In this context, he questions the limitation of the area to be treated (25 cm2). Data on the risk of a single AK developing into SCC within one year is reported in the literature to be 0.025-16%, with rather little known about the risk of progression at this time [9]. But the risk is higher with a larger number of lesions, Prof. Dirschka said. Assuming that the risk of progression of a single AK lesion is 16% per year, this would mean that with 40 lesions the corresponding risk is more than 99% [11].
Pragmatic approach – which therapy goals are targeted?
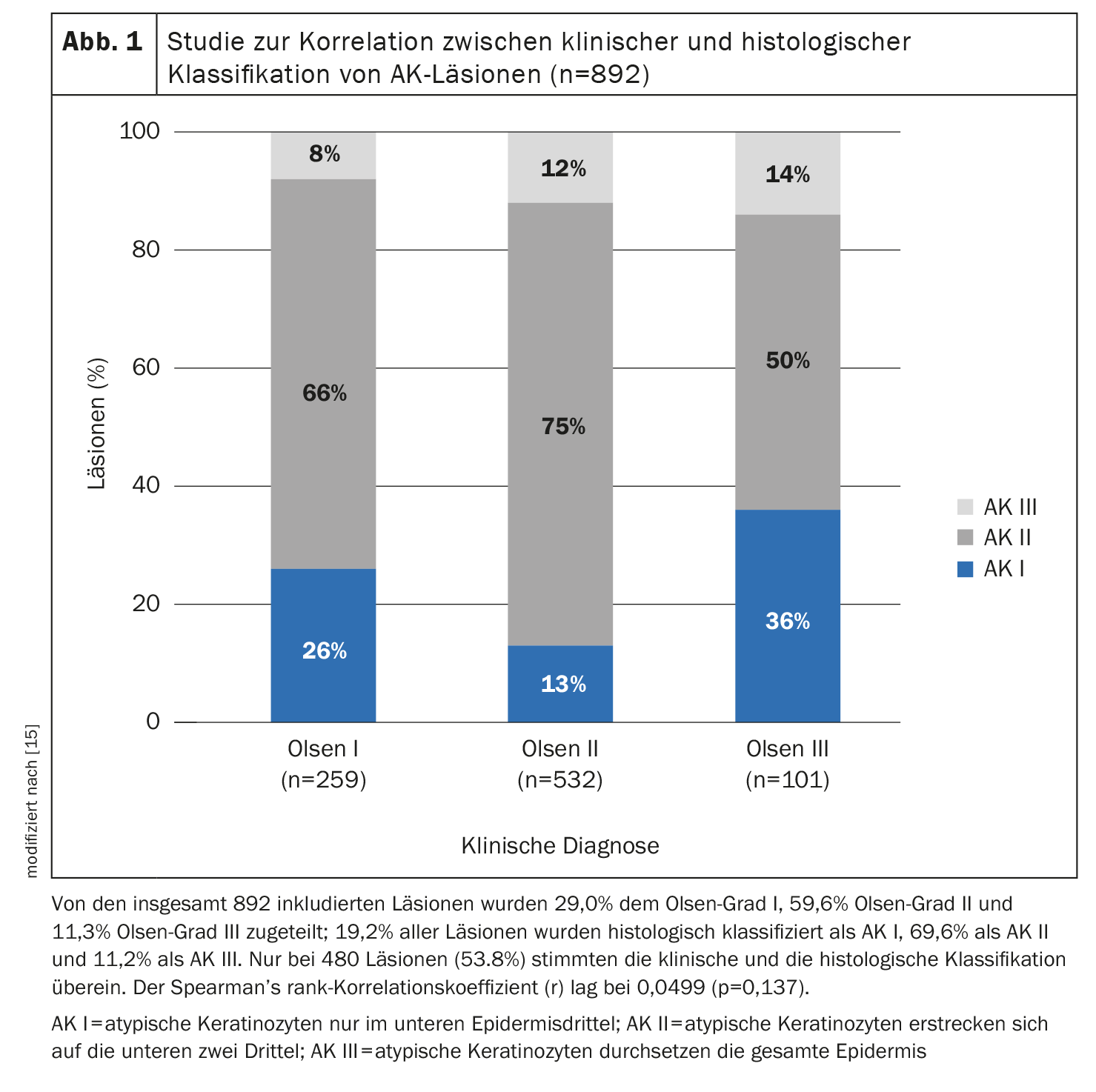
The extent of basal proliferation of atypical keratinocytic cells is an important criterion, said Prof. Dirschka [11]. He does not consider treatment restrictions based on the Olsen classification, which have been introduced in the context of clinical trials, to be useful for everyday clinical practice. First, in one study, Olsen classification was relatively poorly correlated with histopathologic classification of AK lesions (lesion thickness) (Fig. 1), and second, there were Olsen I lesions with much hyperproliferation and Olsen III lesions with little hyperproliferation. From his point of view, the localization of the areas is also outdated. It is important not to focus exclusively on the face and scalp, but also on the extremities, he said. Finally, the speaker suggests that complete freedom from lesions is a rather unrealistic treatment goal in field cancerization and that one should rather aim for a reduction of the AKASI score instead. In addition, treatment-resistant lesions should be given special attention, as they are potentially dangerous.
Literature:
- Ferrándiz C, et al.: EPIQA Study Group; Prevalence of actinic keratosis among dermatology outpatients in Spain. Actas Dermosifiliogr 2016; 107(8): 674–680.
- Flohil SPC, et al.: Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam Study. J Invest Dermatol 2013; 133(8): 1971–1978
- Eder J, et al.: Prevalence of actinic keratosis among dermatology outpatients in Austria. Br J Dermatol 2014; 171(6): 1415–1421.
- Criscione VD, et al.: Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer 2009; 115(11): 2523–2530.
- “Biology of AK,” Symposium 8: Management of actinic keratosis and field cancerization, Prof. Dr. Nicole Kelleners-Smeets, EADO Annual Meeting, April 20-22, 2023.
- Thomson J, et al.: The Genomic Landscape of Actinic Keratosis. J Invest Dermatol 2021; 141(7): 1664–1674.e7.
- «Imaging technologies as diagnostic tools for AK», Symposium 8: Management of actinic keratosis and field cancerization, Dr. Giuseppe Micali, EADO Annual Meeting, 20–22 April 2023.
- Ahlgrimm-Siess V, et al.: Diagnostischer Nutzen der Konfokalmikroskopie als weiterführende Untersuchungsmethode von Gesichtsläsionen. JDDG 2019; 17(3): 266–274.
- AWMF: S3-Leitlinie Aktinische Keratose und Plattenepithelkarzinom der Haut. Register-Nr. 032-022OL, Version 2.0: https://register.awmf.org, (letzter Abruf 04.07.2023)
- «Treatment Update – AK», Dr. Girish Gupta, Symposium 8: Management of actinic keratosis and field cancerization, EADO Annual Meeting, 20–22 April 2023.
- «New approaches to study design in AK», Prof. Dr. Thomas Dirschka, Symposium 8: Management of actinic keratosis and field cancerization, EADO Annual Meeting, 20–22 April 2023.
- Dirschka T, et al; Athens AK Study Group: A proposed scoring system for assessing the severity of actinic keratosis on the head: actinic keratosis area and severity index. J Eur Acad Dermatol Venereol 2017; 31(8): 1295–1302.
- Dirschka T, et al.: A proposed scoring system for assessing the severity of actinic keratosis on the head: actinic keratosis area and severity index. J Eur Acad Dermatol Venereol 2017; 31(8): 1295–1302.
- Dreno B, et al.: A Novel Actinic Keratosis Field Assessment Scale for Grading Actinic Keratosis Disease Severity. Acta dermato-venereologica 2017; 97(9): 1108–1113.
- Schmitz L, et al.: Actinic keratosis: correlation between clinical and histological classification systems. J Eur Acad Dermatol Venereol 2016; 30(8): 1303–1307.
- Babilas P, Landthaler M, Szeimies RM: Die aktinische Keratose. Hautarzt 2003; 54: 551–562.
- Fu W, Cockerell CJ: The actinic (solar) keratosis: a 21st-century perspective. Arch Dermatol 2003; 139: 66–70.
- Schmitz L, et al.: Actinic keratosis area and severity index (AKASI) is associated with the incidence of squamous cell carcinoma. J Eur Acad Dermatol Venereol 2018; 32(5): 752–756.
- Marks R, Rennie G, Selwood TS: Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet 1988; 1: 795–797.
DERMATOLOGIE PRAXIS 2023; 33(4): 28–30