With the approval of several new drugs, the treatment spectrum for second-line therapy of plasma cell myeloma has been expanded. In parallel, established treatment concepts, predominantly in first-line treatment, were questioned. So what is currently valid?
In the last four years, the approval of several new drugs has expanded the treatment spectrum for second-line therapy of patients with plasma cell myeloma. In parallel, however, established treatment concepts, predominantly in first-line treatment, have been questioned in clinical trials. The resulting results, some of which are contradictory, open up a great deal of room for interpretation. This review aims to provide an inventory of current preferences in myeloma treatment in Switzerland.
In anticipation of the SSMO Swiss Consensus Meeting at the Swiss Oncology and Hematology Congress 2018, ten Swiss myeloma experts were asked in writing about controversial topics in myeloma diagnosis and treatment. During the meeting, the same questions were asked of the physicians present. About 40 participants answered the questions (electronic voting).
SLIM-CRAB
An update of the IMWG criteria for the diagnosis of multiple myeloma was published in late 2014 [1]. The definition of treatment-naive myeloma has been expanded to include the SLIM-CRAB criteria (Overview 1). This is intended to identify patients with a very high probability of rapid progression of smoldering myeloma to treatment-naive multiple myeloma according to classical criteria before the appearance of end-organ damage and to direct them to treatment. This concept has now become established in Switzerland.
Two-thirds of the experts surveyed and 85% of the physicians in the audience reported always considering SLIM-CRAB criteria in myeloma diagnosis. One-third of the experts and 15% of the physicians in the audience survey the SLIM parameters but assess the need for treatment in patients with a positive SLIM-CRAB criterion on an individual basis.

Genetic risk stratification
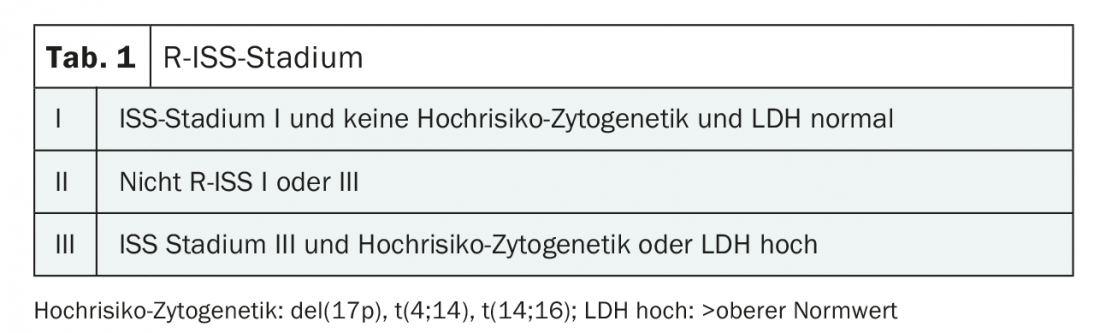
Genetic alterations in myeloma cells are among the most important predictors of patient prognosis. A hyperdiploid chromosome set underlies approximately 50% of myeloma cases and is associated with a “favorable” prognosis (standard risk). Translocations in the IgH locus (e.g., t[4;14], t[11;14]) are correlated with different aggressiveness of myeloma, depending on the fusion partner. Additional secondary genetic alterations are usually prognostically unfavorable (del17p, gain1q, deletion 1p, etc.) [2]. The three best-established cytogenetic alterations with unfavorable risk profiles (del[17p], t[4;14], and t[14:16]), together with albumin and β2-microglobulin in the revised international scoring system (R-ISS) (Table 1) and together with LDH level at diagnosis, allow prognostic differentiation in terms of progression-free survival and overall survival. ESMO and NCCN recommend performing cytogenetics and FISH at diagnosis.
These recommendations are also followed in Switzerland: Half of the responding physicians perform genetic testing in all patients at diagnosis, the other half refrain from genetic testing in elderly and comorbid patients.
High-dose chemotherapy with autologous stem cell transplantation
The value of high-dose chemotherapy with autologous stem cell transplantation (HDT-ASCT) in first-line therapy has been reviewed in several randomized trials in recent years [3,4]. There was significantly better progression-free survival for HDT-ASCT compared with first-line “conventional” therapy (VRD, VMP), but without prolongation of OS. The results of the American StaMINA trial were presented at ASH 2016: Here, no PFS benefit was shown for patients who received HDT-ASCT in the first-line therapy [5]. However, due to various discussion points in the study design of the StaMINA study, these results should be interpreted with caution. The advantage of primary high-dose therapy over conventional myeloma therapy in terms of overall survival was demonstrated before the introduction of proteasome inhibitors into conventional therapy. Formally, in the era of proteasome inhibitors, none of the randomized trials has been able to show an OS benefit for HDT-ASCT in first-line versus conventional therapy, as the follow-up time of the respective trials is currently still too short. Therefore, formally, the use of high-dose therapy would also be justifiable only in the event of a relapse.
However, high-dose chemotherapy continues to have its place in the first-line treatment of myeloma patients. 100% of responding physicians consider HDT-ASCT to be the standard of care in this situation.
Tandem transplantation
Double high-dose chemotherapies with ASCT have been performed since the 1990s. Tandem transplantation results in deeper remission compared to single transplantation and has been associated with prolonged PFS in many studies. Prolongation of OS has not yet been shown for tandem transplantation in the era of induction therapy with proteasome inhibitors or IMIDs. The EMN02/HOVON95 trial incorporated a comparison of tandem vs. single transplant into the study design. Tandem transplantation resulted in significantly better PFS and OS for the entire study population in this trial. The difference was particularly pronounced for patients with high-risk cytogenetics (t[4;14], t[14;16], del[17p]) or R-ISS II + III [6].
Risk-adapted tandem ASCT in first-line treatment is favored by 98% of physicians according to our survey.
Induction therapy before ASCT
Bortezomib-based triplet therapies (so far mostly in combination with cyclophosphamide or thalidomide) for induction have been the international standard for several years. The combination of bortezomib with an alkylane (VCd) is inferior to the combination with an IMID (VTd) in terms of response and remission quality before high-dose therapy [7]. The combination of bortezomib with lenalidomide and dexamethasone has shown a high response rate in several studies and is less neurotoxic than VTD. Randomized comparative studies were not performed. Since 2017, lenalidomide has been approved in Switzerland for first-line treatment of patients who do not qualify for ASCT. This has led to the “easier availability” of lenalidomide even in the setting of induction before ASCT.
Lenalidomide is currently the favored combination partner of bortezomib and dexamethasone (VRD) in induction before HDT-ASCT. For 85% of responding physicians, VRD is the first choice, 12% favor another bortezomib-based triplet combination, and 2% rely on a nonbortezomib-based induction.
Consolidation according to ASCT
Further tumor reduction is achieved with consolidation (two to four cycles of a combination analogous to induction) after HDT-ASCT, which has been associated with prolonged PFS in many studies. This was reconfirmed in the EMN02 trial with a second randomization after ASCT (two cycles of VRD vs. no consolidation), but an OS benefit was not shown. In contrast, the StaMINA trial (no consolidation vs. second ASCT vs. 4× VRD) showed no benefit for patients who received consolidation. The value of consolidation after HDT-ASCT remains unclear.
This is also reflected in the survey results. For 66% of physicians, consolidation after HDT-ASCT is standard. 19% consolidate only patients with high-risk features. 15% generally do not consolidate.
Maintenance therapy
Randomized trials have demonstrated a PFS benefit for patients receiving maintenance therapy with lenalidomide (IFM CALGB). In a meta-analysis with data from over 1200 patients, lenalidomide maintenance was associated with an OS benefit of over two years [8]. The data for bortezomib maintenance is less robust. However, patients with high-risk cytogenetics appear to benefit most from bortezomib maintenance [9].
The optimal duration of lenalidomide maintenance has not been defined. In most studies, maintenance was planned until progression. Median duration of maintenance with lenalidomide was 30 months (early cessation of maintenance in the IFM 2005-02 trial due to increased incidence of secondary malignancies).
For 80% of the Swiss experts interviewed, lenalidomide maintenance is standard for all patients after HDT-ASCT. 20% make the use of maintenance dependent on response to induction therapy and prescribe maintenance therapy only for patients who have not achieved CR or VGPR.
Maintenance with lenalidomide is indicated for 70% of experts for all patients. 30% of experts surveyed differentiate by genetic risk profile and favor bortezomib in maintenance for patients with high-risk cytogenetics.
Duration of maintenance therapy: 50% of physicians favor maintenance until re-progression, 50% favor lenalidomide maintenance therapy limited to (one to) two years.
First-line treatment of patients who do not qualify for HDT-ASCT.
Bortezomib-based triple combinations (VMP, VCD), usually given for six to nine cycles, were the therapy of choice for elderly or comorbid patients until the first-line approval of Revlimid®/dexamethasone. The three-arm randomized FIRST trial compared Rd to MPT for 18 months with Rd to progression. Continuously given Rd results in significantly longer PFS compared with Rd for 18 months, but without an OS benefit [10]. Patients with high-risk cytogenetics appear to benefit from bortezomib-based therapy [11].
When asked whether the choice of treatment protocol is influenced by cytogenetic risk stratification, 60% answered yes: a bortezomib-based protocol is used in patients with high-risk cytogenetics. 10% of physicians choose Rd regardless of cytogenetics and 20% choose a bortezomib-based combination for first-line treatment.
Among bortezomib-based protocols, VMP is slightly preferred at 55% compared with VCD at 45%. The combination of VMP plus daratumumab has not yet been used.
If Rd is used, continuous administration (55%) was mentioned more frequently compared with fixed duration of therapy (45%).
Second-line therapy
Carfilzomib, daratumumab, elotuzumab, and ixazomib in combination with lenalidomide and bortezomib, respectively, are approved for second-line therapy. There are no studies that directly compare these new substances.
When asked about preference in second-line therapy, a choice could be made between three options: KRd was mentioned most frequently with 70%, DRd or DVd reached 20% agreement, and 10% would prefer another therapy.
Take-Home Messages
- The application of the SLIM-CRAB criteria has become established.
- Genetic characterization of myeloma at diagnosis is part of the initial work-up in Switzerland.
- Genetic risk stratification in the treatment of patients is practiced by a large proportion of physicians.
- The treatment of younger myeloma patients in Switzerland is consistent: high-dose chemotherapy and autologous stem cell transplantation remain an undisputed part of first-line treatment. Consolidation for further tumor load reduction is practiced by the majority. Maintenance therapy is standard.
- In elderly patients, bortezomib-based treatment continues to be preferred; Revlimid® dexamethasone has not (yet) gained acceptance.
- Carfilzomib- and daratumumab-based triple combinations have become established in second-line therapy.
Literature:
- Rajkumar V, et al: International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15: e538-548S.
- Morgan GJ, Walker BA, Davies FE: The genetic architecture of multiple myeloma. Nat Rev Cancer 2012; 12(5): 335-348.
- Cavo M, et al: Autologous stem cell transplantation versus bortezomib-melphalan-prednisone for newly diagnosed multiple myeloma: second interim analysis of the phase 3 EMN02/HO95 study. Blood 2017; 130(Suppl 1): 397.
- Attal M, et al: Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017; 376: 1311-1320.
- Stadtmauer EA, et al.Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 – StaMINA Trial). Blood 2016; 128(Suppl 1): LBA-1.
- Cavo M, et al: Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/HO95 study. Blood 2017; 130(Suppl 1): 401.
- Moreau P, et al: VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood 2016; 127(21): 2569-2574.
- McCarthy PL, et al: Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol 2017; 35: 3279-3289.
- Sonneveld P, et al: Bortezomib Induction and Maintenance Treatment in Patients With Newly Diagnosed Multiple Myeloma: Results of the Randomized Phase III HOVON-65/GMMG-HD4 Trial. J Clin Oncol 2012; 30: 2946-2955.
- Facon T, et al: Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood 2018; 131(3): 301-310.
- Larocca A, et al: Impact of bortezomib- or lenalidomide-based induction treatment on high-risk cytogenetic transplant-ineligible patients with newly diagnosed multiple myeloma enrolled in the Gimema-MM-03-05 and EMN01 trials. Blood 2017; 130(Suppl 1): 744.
InFo ONCOLOGY & HEMATOLOGY 2018; 6(6): 18-20.