Sparing and targeted antibiotic use helps minimize the risk of antibiotic resistance. Any antibiotic administration also leaves collateral damage in our microbiome. In general, therapy should be as short as necessary and as high a dose as possible.
Antibiotics helped make infectious diseases that used to be severe and often fatal treatable, thus losing their former horror. For example, compared with the pre-antibiotic era, mortality from pneumonia was reduced from about 23% to about 7%, mortality from endocarditis was reduced from 100% to 25%, and mortality from bacterial meningitis was reduced from >80% to <20% [1]. The introduction of antibiotics brought a surge of development to modern high-performance medicine. Without effective antibiotics, intensive care, surgery, especially endoprosthetics and transplantation, and chemotherapy are inconceivable [2].
Antibiotic resistance
After the introduction of a new antibiotic, it usually takes only a few years for bacteria to become resistant to it. Antibiotics exert selection pressure on pre-existing resistant bacterial variants, which thus have a survival advantage over susceptible bacteria and become enriched.
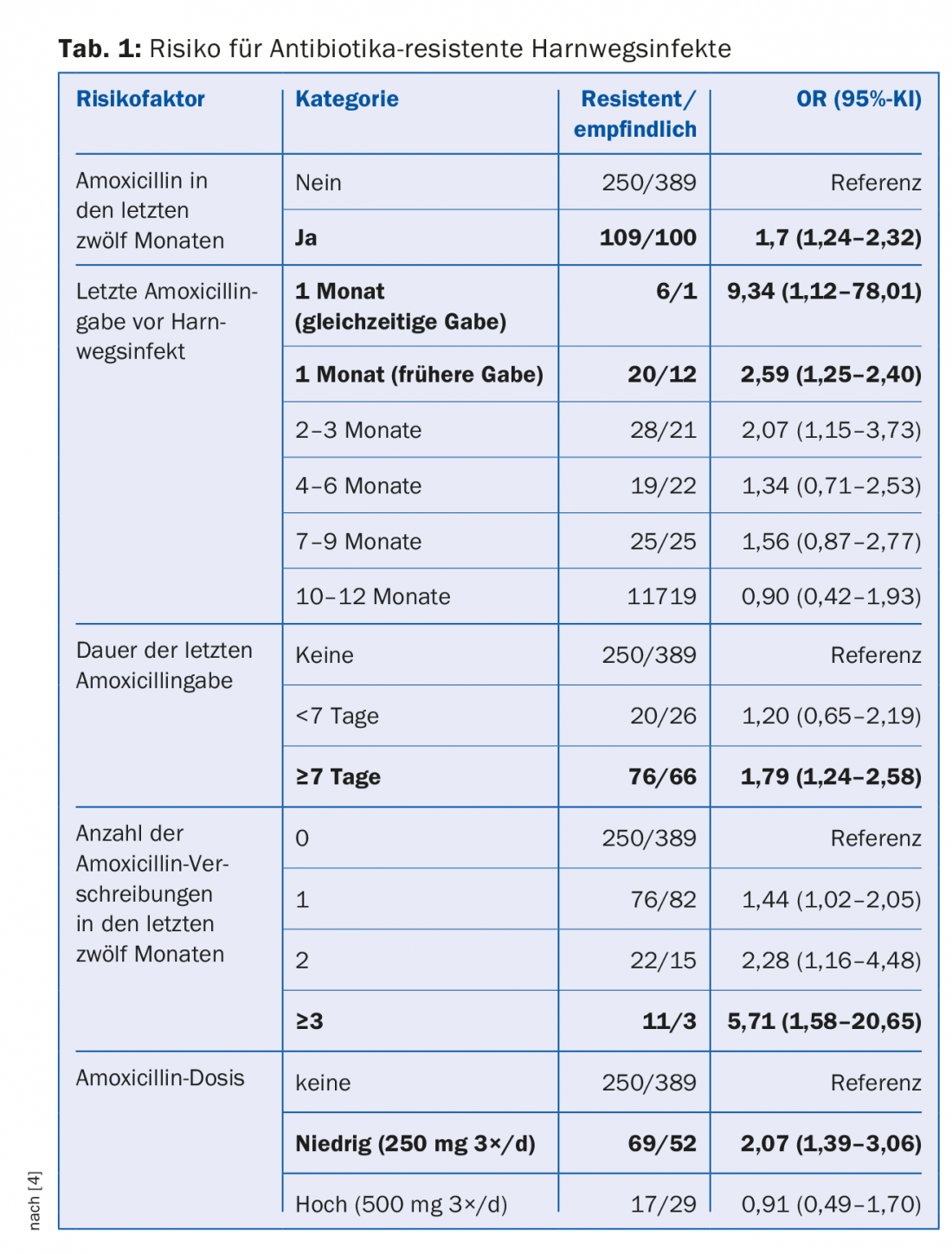
Antibiotic resistance has been present in nature for millions of years, but it is clearly antibiotic use in agriculture and medicine, ultimately humans, that is the driving force. The more antibiotics prescribed in a population, the higher the risk for antibiotic-resistant bacteria [3]. In a case-control study of patients with urinary tract infections, several risk factors emerged: frequent antibiotic administration, long duration of therapy, low dose (Table 1) [4]. The risk of colonization with penicillin-resistant pneumococci increased by 4% in Australian children for each day they had received β-lactam antibiotics in the previous six months [5]. Approximately 60-90% of human antibiotics are prescribed in the outpatient setting and of these, approximately 75-85% are prescribed for commonly viral respiratory infections. However, globally, about 80% of all antibiotics are used in livestock production, primarily as “growth promoters” for livestock fattening.
Antibiotic resistance can arise and spread in several ways. They may be intrinsic (i.e., a bacterium is always resistant to that antibiotic), arise de novo by spontaneous mutation and become selected due to selection pressure by the antibiotic, be transferred from one bacterium to another by horizontal gene transfer (e.g., by means of plasmids on which different resistance genes are often present simultaneously), and also be passed between patients by poor (hand) hygiene.
Antibiotic resistance has relevant consequences such as increased mortality, longer hospital stays and higher costs (overview 1) . Grim projections predict an increase from about 25,000 deaths in the EU and about 700,000 worldwide due to infections with antibiotic-resistant pathogens in 2014 to up to 390,000 and 10 million, respectively, in 2050. This raises fears of a regression to a pre-antibiotic era with untreatable infections – now a reality in many countries – and indirect costs of an unimaginable $100 trillion (amr-review.org).

In Switzerland, data on antibiotic resistance are collected with national surveillance and are available in an interactive database at www.anresis.ch. Here, considerable success has been achieved in containing methicillin-resistant Staphylococcus aureus (MRSA). Less than 3% of pneumococci are resistant to penicillin, in part due to pneumococcal vaccination in children. Unfortunately, antibiotic resistance is steadily spreading among Gram-negative pathogens, particularly by extended-spectrum beta-lactamases (ESBLs), which are increasingly responsible for abdominal and urinary tract infections. For example, about 10% of all Escherichia coli in Switzerland are ESBL-formers and about 20% are resistant to ciprofloxacin.
Effects of antibiotics on the microbiome.
Apart from resistance selection, adverse side effects, allergic reactions and drug costs, each antibiotic use has a relevant impact on our microbiota (totality of all organisms in our body) and our microbiome (microbiota as well as its genome) lasting up to several years [6]. The best known is the predisposition after antibiotics to Clostridium difficile-associateddiarrhea, but antibiotics also increase the risk of sepsis in the course, development of obesity, diabetes mellitus, asthma, and allergies, among others [7]. The greatest influence is on the developing microbiota of the child, which is why care should be taken to use antibiotics as restrictively as possible, particularly in pregnant women and children (especially in the first two years of life) [8].
In particular, anaerobic bacteria of the site flora in the colon contribute to colonization resistance to pathogens, which is why antibiotic therapy directed against anaerobes predisposes to other infections. It is interesting to note that after travel to an endemic area, the risk of new colonization with multidrug-resistant Enterobacteriaceae compared with healthy travelers was twice as high in individuals who had diarrhea during the trip and more than four times as high in individuals who took a β-lactam antibiotic during the trip (dysbiosis of intestinal flora) [9]. This is an important argument against overly generous antibiotic prophylaxis against traveler’s diarrhea.
Basic rules for the use of antibiotics
In any antibiotic therapy, the following aspects should be considered:
Indication: Any antibiotic administration should be done only after strict indication. The use of biomarkers can be helpful for this. Because the risk of bacterial infection correlates with the level of procalcitonin (PCT), without increased risk of complications, the use of PCT algorithms can reduce antibiotic use by 35-45% in patients with exacerbation of chronic obstructive pulmonary disease (COPD) and by approximately 65% in those with upper respiratory tract infections [10]. Antibiotics should be avoided in patients with typical viral symptoms in upper respiratory tract infections or acute bronchitis (conjunctivitis, rhinitis, arthralgia, exanthema). A British study showed that restrictive use of antibiotics for upper respiratory tract infections could save more than 2000 antibiotic prescriptions over ten years in a practice with 7000 patients per year [11]. Feared complications occur very rarely as a result (approximately ten episodes of pneumonia and less than one peritonsillar abscess over ten years). It should be noted that the upper and lower respiratory tracts are not sterile and therefore bacterial detection does not automatically indicate antibiotic therapy.
Asymptomatic bacteriuria should be sought only in pregnancy and before urologic procedures involving mucosal injury, and only then should it be treated with antibiotics. Otherwise, antibiotic therapy for asymptomatic bacteriuria is associated with an increased risk of developing pyelonephritis (again, presumably via dysbiosis of the microbiota) and with increased development of resistance [12,13]. The same is true for asymptomatic indwelling catheter users, who have a colonization rate of approximately 8%/d, which should not lead to antibiotic therapy in the absence of clear symptoms.
Acute sinusitis usually has a viral etiology. Only 0.5-2% of adults and up to 5% of children develop a bacterial superinfection. Antibiotics are recommended only for persistent symptoms or lack of improvement after at least ten days, severe symptoms (fever ≥39°C or purulent sputum) for at least three to four days, or worsening or biphasic course after at least three to four days. The treatment of choice is amoxicillin 1 g every eight hours for five to seven days, or possibly amoxicillin/clavulanic acid 1 g every twelve hours for five to seven days in severe cases or with risk factors.
Immediate antibiotic therapy is indicated only for bilateral acute otitis media (AOM) in children younger than two years or for perforated AOM. Otherwise, “watchful waiting” is recommended for 24-48 hours in children under two years of age or 48-72 hours in those over two years of age, as antibiotic therapy has little effect on symptom duration or complication rate in AOM, which is usually initially viral. Therapy of choice is amoxicillin, with amoxicillin/clavulanic acid in case of relapse or lack of response after 72 hours.
In patients older than three years, the Centor score (one point each for exudate on tonsils; fever >38°C; painful, swollen cervical anterior lymph nodes; absence of cough) should be calculated if nonviral pharyngitis is suspected. Only if this yields ≥3 points should a rapid test for Streptococcus pyogenes be performed. And only if positive, antibiotic therapy should be started within nine days to minimize the risk of acute rheumatic fever. Therapy of choice is amoxicillin 1 g every twelve hours for six days (so far, S. pyogenes is always sensitive to penicillin, but amoxicillin has a higher bioavailability than penicillin).
Several recent studies have shown that antibiotic therapy is no better than placebo for uncomplicated acute diverticulitis in terms of symptom relief, complication rate, need for surgery, and recurrence rate [14].
In acute gastroenteritis (vomiting diarrhea/watery diarrhea) without fever with/without travel history, symptomatic therapy is usually sufficient. Antibiotics should be used only in cases of bloody or febrile diarrhea or systemic toxicity.
Pathogen spectrum: Any empiric antibiotic therapy should take into account the expected pathogen spectrum. Both the most common pathogens and, in some cases, rare pathogens associated with a particularly severe course must be treated. Depending on the infection, microbiological pathogen diagnostics are indicated. If a pathogen is detected, therapy should be tapered (de-escalation). This is done in particular to minimize potential collateral damage to the microbiome and selection of antibiotic resistance. For example, it has been shown that patients with bacteremic pneumococcal pneumonia had lower mortality when de-escalated to penicillin or amoxicillin monotherapy during the course [15].
Mode of administration: antibiotics with high oral bioavailability should be administered primarily orally, e.g., trimethoprim-sulfamethoxazole, metronidazole, clindamycin, quinolones. Exceptions are patients in whom enteral absorption is impaired or infections that require very high levels at the site of action (e.g., very severe infections, endovascular infections, bone infections initial, CNS infections).
Dosage: The dose depends on the minimum inhibitory concentration (MIC) of the pathogen, the germ load, the severity of the infection, the expected tissue levels (mostly low in prostate, CNS) and the therapeutic range of the antibiotic (high for β-lactam antibiotics, low for aminoglycosides). The initial dose should be selected as high as possible, since the highest germ load is present here and a loading dose is often also required in order to achieve sufficient active levels as quickly as possible. Optimal dosages are often higher than recommended in the compendium (see, for example, www.guidelines.ch). In case of hepatic or renal insufficiency, an extension of the interval may be necessary, but the loading dose remains the same. β-lactam antibiotics (penicillins, cephalosporins) have a time-dependent effect, in which the active level should remain above the MIC of the pathogen for as long as possible by dosing as frequently as possible (short intervals). In contrast, macrolides, aminoglycosides or quinolones act in a concentration-dependent manner and achieve an optimal effect at high peak concentrations. For these antibiotics, the highest possible dose with a long dose interval should be given.
Duration: The duration of therapy should be as long as necessary and as short as possible. The background to this is that as little selection pressure as possible should be exerted for the emergence and spread of antibiotic resistance. This concept is also gaining international attention, and in the majority of infections relevant to family practice, short durations of therapy are now well established. Reference should be made here to two very good overview articles [16,17]. Overview 2 gives an overview.

Allergy history: of all antibiotic allergies, penicillin allergy is the most commonly reported in patients. However, probably less than 10% of these patients have true penicillin allergy and in less than 2% truly penicillin-allergic patients exhibit cross-allergy to third-generation cephalosporins [18]. It should be noted that penicillins and other β-lactam antibiotics are usually the most effective class of substances, and their withholding due to a perceived allergy leads to increased morbidity and mortality in patients “labeled” in this way. Therefore, allergies should always be differentiated from adverse reactions and suspected antibiotic allergy should be clarified allergologically.
Repair of the damaged microbiota: Just as a surgeon is responsible for his or her wound, the consequences (especially resistance selection and collateral damage in terms of the microbiome) should be considered whenever antibiotics are prescribed. The goal should be to minimize or “make up” for them. However, future studies must first provide evidence on which probiotic is best in which situation. Various Lactobacillus and Bifidobacillus species contained in various commercially available drinking yogurts or dietary supplements are recommended to restore microbiota damaged by antibiotics [6].
Take-Home Messages
- 60-90% of antibiotics are prescribed in the office and about 75-85% of these are for mostly viral respiratory infections.
- The following aspects must be taken into account for every antibiotic therapy: Indication, expected pathogen spectrum, mode of application, dosage, duration.
- Using them as sparingly and selectively as possible helps to minimize the risk of developing antibiotic resistance, side effects, allergies and costs.
- Any antibiotic administration leaves collateral damage in our microbiome with multiple and still incompletely known effects on the risk of diverse diseases (Clostridium difficile-associated diarrhea, sepsis, obesity and metabolic syndrome, allergies, etc.).
- Rule of thumb for antibiotic therapy: as short as necessary and as high a dose as possible.
Literature:
- Spellberg B, et al: Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 2011; 52(Suppl 5): S397-428.
- Marston HD, et al: Antimicrobial resistance. JAMA 2016; 316(11): 1193-1204.
- Albrich WC, Monnet DL, Harbarth S: Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis 2004; 10(3): 514-517.
- Hillier S, et al: Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case-control study. J Antimicrob Chemother 2007; 60(1): 92-99.
- Nasrin D, et al: Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ 2002; 324(7328): 28-30.
- Kahlert C, Müller P: Microbiome – the discovery of an organ. Swiss Med Forum 2014; 14(16-17): 342-344.
- Willing BP, Russell SL, Finlay BB: Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 2011; 9(4): 233-243.
- Blaser MJ: The theory of disappearing microbiota and the epidemics of chronic diseases. Nature reviews Immunology 2017; 17(8): 461-463.
- Ruppe E, et al: High Rate of Acquisition but Short Duration of Carriage of Multidrug-Resistant Enterobacteriaceae After Travel to the Tropics. Clin Infect Dis 2015; 61(4): 593-600.
- Schuetz P, et al: Less is more: individualized antibiotic therapy by measuring procalcitonin. Swiss Med Forum 2012; 12(46): 887-892.
- Gulliford MC, et al: Safety of reduced antibiotic prescribing for self-limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ 2016; 354: i3410.
- Cai T, et al: The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis 2012; 55(6): 771-777.
- Cai T, et al: Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis 2015; 61(11): 1655-1661.
- Chabok A, et al: Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. The British journal of surgery 2012; 99(4): 532-539.
- Cremers AJ, et al: Effect of antibiotic streamlining on patient outcome in pneumococcal bacteraemia. J Antimicrob Chemother 2014; 69(8): 2258-2264.
- Llewelyn MJ, et al: The antibiotic course has had its day. BMJ 2017; 358: j3418.
- Dawson-Hahn EE, et al: Short-course versus long-course oral antibiotic treatment for infections treated in outpatient settings: a review of systematic reviews. Family practice 2017; 34(5): 511-519.
- Trubiano JA, Adkinson NF, Phillips EJ: Penicillin Allergy Is Not Necessarily Forever. JAMA 2017; 318(1): 82-83.
HAUSARZT PRAXIS 2018; 13(4): 11-14