In the course of the last decades, the range of parameters for the clarification of neurological diseases has increased disproportionately compared to other fields of immunology. Many diseases whose pathogenesis was not known or which had been labeled as psychiatric are in fact autoimmune.
In the course of the last decades, the range of parameters for the clarification of neurological diseases has increased disproportionately compared to other fields of immunology. Many diseases whose pathogenesis was not known or which had been labeled as psychiatric are in fact autoimmune. These autoantibodies are divided into two groups that differ with respect to pathogenesis, clinic, tumor association, therapy, prognosis, and diagnosis [1]. One antibody recognizes intracellular antigens, is associated with paraneoplastic syndromes, and is therefore also called onconeuronal antibody. The others are directed against extracellular domains of receptors or channels. They cause autoimmune encephalitides, respond better to immunotherapy, and show a better prognosis with early therapy, making correct diagnosis important. Antibodies against GAD (glutamate decarboxylase) occupy an intermediate position. The antigen sits presynaptically intracellularly in the synaptic vesicles, but is released into the synaptic cleft during signal transduction [2].
Antibodies against intracellular antigens
In the context of tumors, neurological symptoms may occur that are not triggered by the tumor itself or the therapy. When a tumor, e.g., small cell lung carcinoma derived from neuroectoderm, expresses ectopic neuronal proteins [3], the immune system activates cytotoxic T cells for defense, which may secondarily damage neurological structures. In two-thirds of patients, the tumor is not yet known at the onset of neurologic symptoms. Paraneoplastic antibodies can be detected in the blood, which are not pathogenic themselves but are important for diagnosis [4]. Usually, these antibodies are determined by immunoblot. Serum is sufficient, testing of CSF does not provide additional information. It is essential that the laboratory retest any positive result using indirect immunofluorescence on brain tissue (Fig. 1) [5].
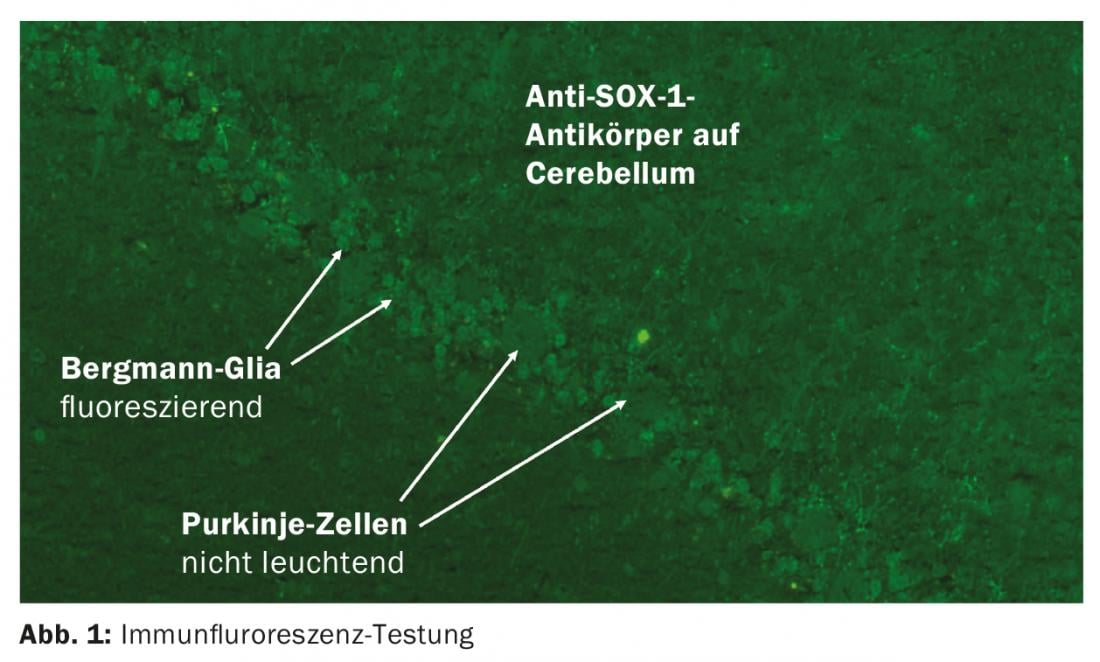
Among the paraneoplastic antibodies, there are well-characterized ones that are known to have a malignancy behind them in >95% of cases. Especially in children there are also cases without tumor, so that in this age group these antibodies should be searched for in case of corresponding symptoms [6]. In the case of partially characterized paraneoplastic antibodies, the predictive value with respect to tumor is unclear due to the still insufficient database [5]. Anti-Tr and anti-Sox-1 appear to be particularly relevant as indicative of malignancy. However, paraneoplastic antibodies cannot be detected in all patients, so antibody detection is not obligatory for diagnosis. Tumor search can be more targeted by considering the clinical syndrome, antibody, and information on age, sex, and nicotine history.
Table 1 lists the antibodies that are routinely determined. The most common neurologic manifestations and associated malignancies are listed.
Antibodies against surface antigens
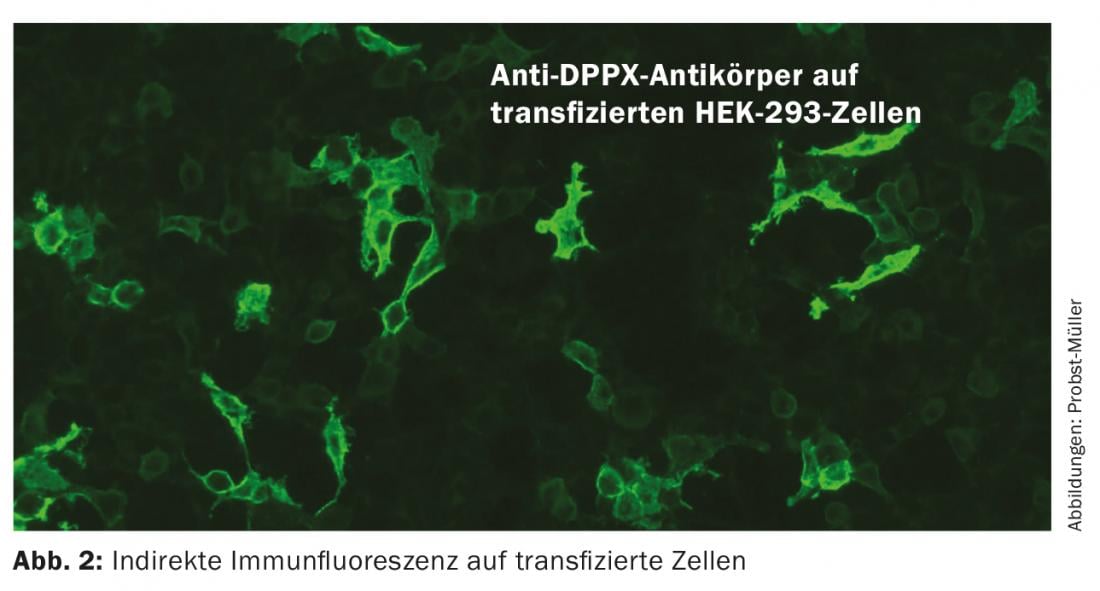
Antibodies against channels and receptors recognize complex epitopes in contrast to the linear epitopes of intracellular antibodies. The antibodies directly trigger the symptoms of the disease by causing electrophysiological changes, disruption of synaptic transmission and neuronal plasticity. Possible mechanisms of action include crosslinking and protein internalization. Patients often respond well to immunotherapy because when the antibody is removed, the proteins can resume their position on the membrane and perform their function. The course is often monophasic, but recurrences are possible. These antibodies are much less likely to be paraneoplastic. Detection of these antibodies is performed by indirect immunofluorescence on transfected cells (Fig. 2), and for the detection of antibodies against VGCC (voltage-gated calcium channels) also by RIA (radioimmunoassay). Immunoblots are not suitable because they denature the proteins, which means that they are no longer recognized by the antibodies. Inflammatory changes in CSF and MRI may be absent, so diagnosis of these conditions can be difficult. An overview of the most important antibodies can be found in Table 2.
Limbic encephalitis (LE): the typical symptoms of LE are memory loss, psychiatric abnormalities, and epileptic seizures. In the absence of the latter, the condition was often considered psychiatric in the past. The CSF often, but unfortunately not always, shows inflammatory changes: lymphocytic pleocytosis, protein elevation, mild barrier disturbance, and possibly oligoclonal bands. Mediotemporal abnormalities may be present on EEG, and MRI may show inflammation of the hippocampus. In addition, patients should be screened for antibodies associated with LE. Ideally, serum and, if possible, CSF should be tested. A wide variety of antibodies have been described in LE (Tab. 2). Other mammals can also become ill. The seizure that led to the drowning of Knut the polar bear was triggered by NMDA receptor antibodies.
NMDA receptor AK encephalitis: NMDA receptors are ion channels activated by glutamate. There are many different glutamate receptors in the nervous system. They are important for excitatory nerve impulses. The name NMDA receptor comes from the fact that this channel is activated by the synthetic molecule NMDA. Encephalitis triggered by antibodies to the NMDA receptor [7,8] was originally described in young women or girls with teratoma of the ovary, but other age groups and men are also affected. No teratoma is present in these patients. At the beginning of the disease, psychiatric disorders are in the foreground, then cognitive losses and epileptic seizures occur. Impaired consciousness and autonomic dysregulation may lead to admission to the intensive care unit. In most cases, antibody detection from serum is sufficient. Rarely, only the CSF is positive, so if strongly suspected, the CSF should also be examined.
Encephalitis due to antibodies against LGI1 and CASPR2: Voltage-gated potassium channels (VGKC) exist throughout the brain. They serve to restore the membrane potential during hyperpolarization. Using RIA, antibodies against VGKCs can be found in patients with neuromyotonia and limbic encephalitis. It is now known that the antibodies are not directly directed against the VGKC, but against two associated proteins, LGI1 and CASPR2 [9]. Patients with these antibodies are predominantly male. They mainly suffer from limbic encephalitis with extralimbic symptoms such as neuromyotonia with painful muscle spasms, movement disorders, sleep disturbances and serum hyponatremia. Depending on the antibody, these symptoms occur with varying frequency. Faciobrachial dystonic seizures occur only in LGI1-positive patients. These are unilateral seizures with grimacing and dystonia of the ipsilateral arm that last less than three seconds but can occur up to hundreds of times daily. These seizures are triggered by movements, emotions and loud noises. Because these seizures occur before limbic encephalitis with cognitive deficits develops, they allow early diagnosis and treatment. Dysautonomia is dangerous with, among other things, bradycardia and sudden cardiac death. This mainly affects CASPR2-positive patients.
Double-negative patients are those who have antibodies to the VGKC but are negative for antibodies to LGI1 and CASPR2. Recent studies show that these antibodies bind to intracellular parts of VGKC or the snake venom dendrotoxin used in RIA, and are not pathogenic. It is found in healthy individuals or in patients with non-autoimmune diseases such as Alzheimer’s disease or Parkinson’s disease. In addition, they are detectable in healthy workers from slaughterhouses exposed to aerosols from animal brains. These data indicate that it is probably sufficient to determine only the antibodies against LGI1 and CASPR2, but not those against the VGKC complex.
Anti-GAD
The enzyme glutamate decarboxylase (GAD) converts glutamate, the main activating neurotransmitter, into γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter, in a single step. The enzyme is found in the nervous system and in the pancreas. Even though GAD is an intracellular molecule, it is released when synaptic vesicles in the brain are shed and can be recognized by autoantibodies. Autoantibodies against GAD occur in diabetes mellitus type I on the one hand, and in neurological diseases on the other hand [10]. In diabetes mellitus, the titers are low. In neurological diseases, the titers are very high. Associated disorders include cerebellar ataxia, limbic encephalitis, and stiff person syndrome (SPS). SPS is a rare disorder that can be either spontaneous or paraneoplastic. A typical feature is an increase in muscle tone that increases over the years. Spontaneous or triggered convulsions occur. Because these spasms are also triggered by emotions, sounds, touch, etc., the condition is often initially dismissed as psychiatric. In the electromyogram, one finds permanent activity, even when the patient tries to relax. If antibodies to GAD are detectable, the SPS is usually not paraneoplastic. Detection of antibodies to amphiphysin (Table 1) involves women with breast carcinoma.
Outlook
The antibodies presented here are only the most important ones. More antibodies will certainly be added in the coming years. It is important to remember this in patients with vague neurological symptoms so that the diagnosis is made early and the patient can be offered the best possible therapy.
Take-Home Messages
- In recent years, a variety of diagnostically and prognostically relevant antibodies have been described that trigger immune-mediated diseases of the central and peripheral nervous system.
- The target antigens of the antibodies are classified according to their localization in membranous and intracellular.
- Antibodies against intracellular antigens such as Hu, Ri, Yo, CV2, SOX-1 are also called onconeuronal, as they usually occur in the context of malignancies. A tumor must be sought. Immunotherapy has very limited promise.
- Antibodies against membrane-bound antigens such as receptors and channels, e.g., NMDA receptors or potassium channels, directly trigger symptoms and lead to autoimmune encephalitides. Patients usually respond well to immunotherapy.
Literature:
- Rachel L, et al: Autoantibody testing in encephalopathies. Practical Neurology 2012; 12: 4-13.
- Bost C, et al: Autoimmune encephalitis in psychiatric institutions: current perspecctives. Neuropsychiatric Disease and Treatment 2016; 12: 2775-2787.
- Onganer PU, et al: Neuronal characteristics of small-cell lung cancer. British Journal of Cancer; 93: 1197-1201.
- Dalmau JO, et al: Paraneoplastic syndromes. Arch Neurol 1999; 56: 405-408.
- www.dgn.org/leitlinien/2383-ll-79-2012-paraneoplastische-neurologische-syndrome
- Honnorat J, et al: Autoimmune limbic encephalopathy and anti-Hu antibodies in children without cancer. Neurology 2013; 80(24): 2226-2232.
- Gresa-Arribas N, et al: Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014; 13(2): 167-177.
- Dalmau J, et al: Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7(12): 1091-1098.
- Binks NM, et al: LGI1, CASPR2 and related antibodies: a molecular evolution of the phenotypes. J Neurol Neurosurg Psychiatry 2017; 0: 1-9.
- Saiz A, et al: Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain 2008; 131: 2553-2563.
InFo NEUROLOGY & PSYCHIATRY 2018; 16(2): 4-8.