In a prospective, non-interventional multi-center study, the treatment effects of calcipotriol/betamethasone dipropionate (Cal/Bet) spray foam in patients with scalp psoriasis were investigated in a “real-world” setting. This topical therapy option was not only convincing in terms of efficacy, but also proved to be well tolerated. This finding is consistent with earlier evidence.
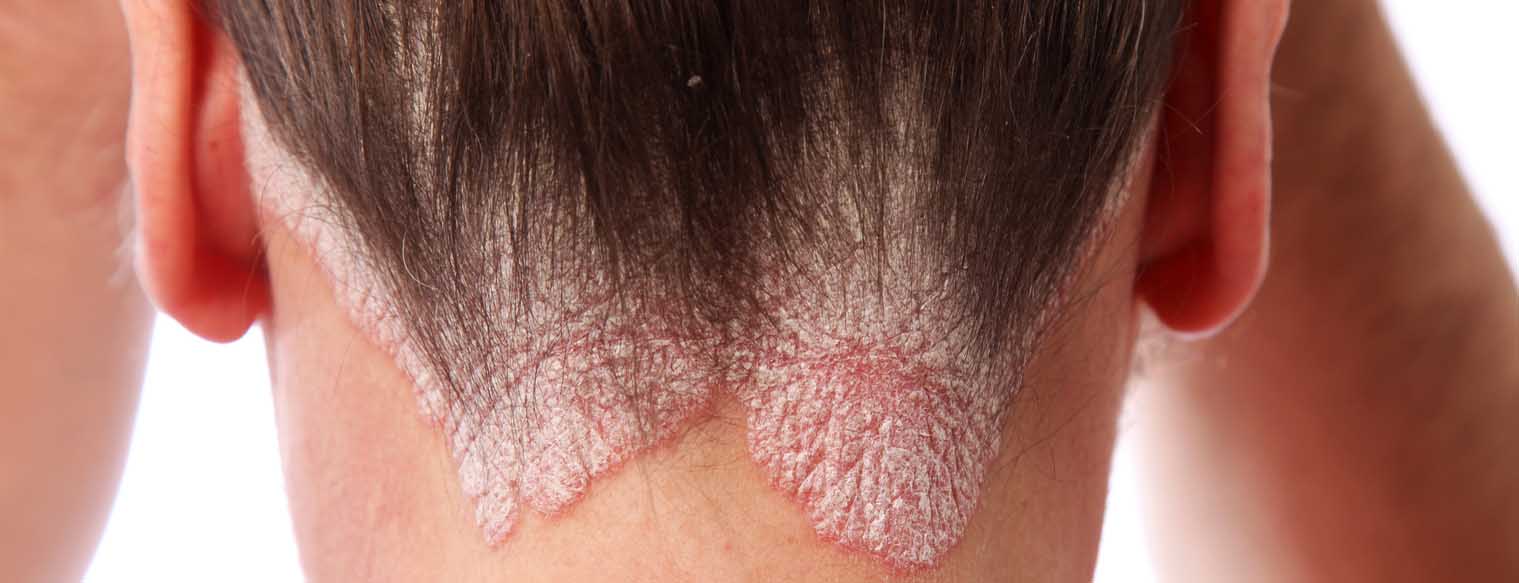
Up to 80% of psoriasis patients have scalp involvement [1,2]. The treatment of capillitium is often a challenge. In addition to efficacy, the most important factor with regard to patient adherence is good acceptance of the formulation of the preparation [3]. Fixed combinations of calcipotriol/betamethasone dipropionate (Cal/Bet) are established topical therapy options for the treatment of psoriasis capitis. Compared to their use as monosubstances, cal/bet fix combinations have additive effects in terms of reducing the hyperproliferation of keratinocytes and inflammatory processes [3,4]. Betamethasone also reduces the local side effects of calcipotriol (lower risk of skin atrophy and burning) [3,4].
| Current Swiss treatment pathway recommends topical therapy with Cal/Bet fixed combinations The Swiss treatment pathway for topical psoriasis therapy published in 2021 points out that the Cal/Bet spray foam (Enstilar®) has repeatedly proven its effectiveness in the treatment of scalp psoriasis [3,8–10,11]. The foam spray formulation has good patient acceptance and proved to be an effective treatment option. The induction therapy (2-4 weeks) is followed by the maintenance phase, during which the frequency of application can be reduced to twice a week. Depending on patient preference, a switch to the Cal/Bet fixed combination in gel form (Daivobet® Gel, Xamiol®) can also be considered [3,11]. The fixed combinations of calcipotriol plus betamethasone dipropionate, which are available as a spray foam and gel, are well tolerated and completely free of water and alcohol. The correct application procedure at the capillitium should be discussed with the patient before starting treatment. |
CAPITIS study underpins evidence base
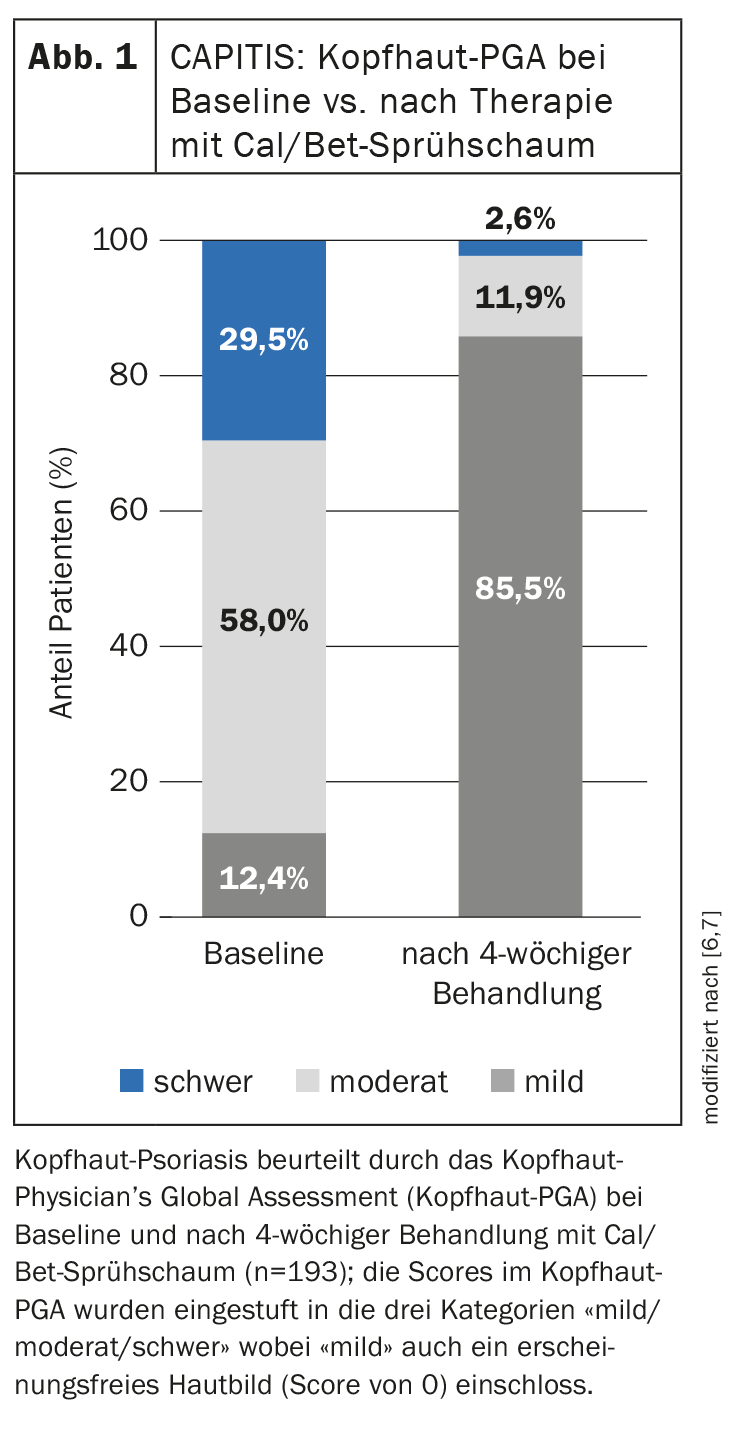
The observational study included 217 adults with scalp psoriasis from 96 centers in Germany [5,6]. 53.4% of patients treated with Cal/Bet spray foam for 4 weeks achieved a scalp BSA <10% and a mild scalp PGA. The mean change in scalp PGA after 4 weeks of treatment with Cal/Bet spray foam is shown in Figure 1 [6]. The proportion of patients with a mild case increased from 12.4% (n=24) to 85.5% (n=165), while the rate of those with a moderate or severe case fell from 58.0% and 29.5% to 11.9% and 2.6% respectively. In addition, 47.6% of patients achieved a PSSI ≤2. A reduction in pruritus and other symptoms (induration, erythema and scaling) was already observed after day 3. The quality of life of patients treated with Cal/Bet spray foam improved from a DLQI of 9.6 (SD ± 5.5) at baseline to 2.8 (± 3.5) after 4 weeks. The proportion of patients with a DLQI 0/1 increased from 3.2% at baseline to 47.9% at the end of the study. Patient satisfaction was high, as reflected in TSQM-9 scores of 74.5 (± 27.1) for efficacy, 72.0 (± 25.2) for ease of use and 77.8 (± 24.2) for overall satisfaction. 97.4% of the treating physicians rated the tolerability of the Cal/Bet spray foam as good or very good and no new safety-relevant signals were reported.
In summary, the results of this observational study confirm the favorable risk-benefit profile of this treatment option in patients with scalp psoriasis.
Congress: DDG Annual Conference
| Abbreviations Scalp-BSA=Scalp-Body Surface Area Scalp PGA = Scalp Physician’s Global Assessment PSSI=Psoriasis Scalp Severity Index DLQI = Dermatology Life Quality Index TSQM=Treatment Satisfaction Questionnaire for Medication |
Literature:
- Schlager JG, et al: Cochrane Database Syst Rev 2016 Feb; 2: CD009687.
- Chan CS, et al: JAAD 2009; 60(6): 962-971.
- Maul J-T, et al: Dermatology 2021; 237: 166-178.
- Segaert S, Ropke M: J Drugs Dermatol 2013; 12(8): e129-137.
- Staubach-Renz P, et al: CAPITIS study, P083. Volume of abstracts, DDG conference, 26-29.04.2023.
- Staubach P, et al: Dermatology 2023; 239(2): 206-216.
- Ortonne J, et al: JEADV 2009; 23(12): 1435-1444
- Paul C, et al: JEADV 2017; 31(1): 119-126.
- Lebwohl M, et al: JCAD 2016; 9(2): 34-41.
- Anderko M, et al: Clin Cosmet Investig Dermatol 2019; 12: 699-705.
- Drug information, www.swissmedicinfo.ch (last accessed 15.08.2023).
DERMATOLOGIE PRAXIS 2023; 33(6): 24 (published on 11.12.23, ahead of print)