Vesicoureteral reflux affects approximately 1% of newborns. A gel composed of dextranomer and hyaluronic acid is a proven minimally invasive treatment option with proven efficacy. The therapy stops the reflux of urine into the ureter very effectively. In studies, treatment proved successful in over 90% of children with VUR grade 2-4.
Deflux® is a minimally invasive treatment used in children with vesicoureteral reflux (VUR) and has been a proven therapy in children with VUR for more than 20 years with over 400,000 procedures [1]. The company Farco-Pharma GmbH has announced that they are taking over the distribution rights for Deflux® for the treatment of children with VUR in Germany, Austria and Switzerland. With the acquisition of the distribution rights, Farco-Pharma further expands its urology portfolio of high-quality gels and instillation solutions [1].
|
Effective and tolerable therapy Deflux® is a viscous injectable gel of dextranomer in a carrier gel that is injected at the site where the ureter connects to the bladder in children with VUR using a minimally invasive procedure. It is an effective and tolerable therapy for VUR that prevents urine reflux into the kidneys [2]. Connective tissue cells gradually grow into the gel injected at the junction of the ureter and bladder, and new tissue is formed. Deflux® consists of dextranomer and hyaluronic acid. The gel is available in glass syringes with Luer lock connector and 1 ml content. |
High success rate
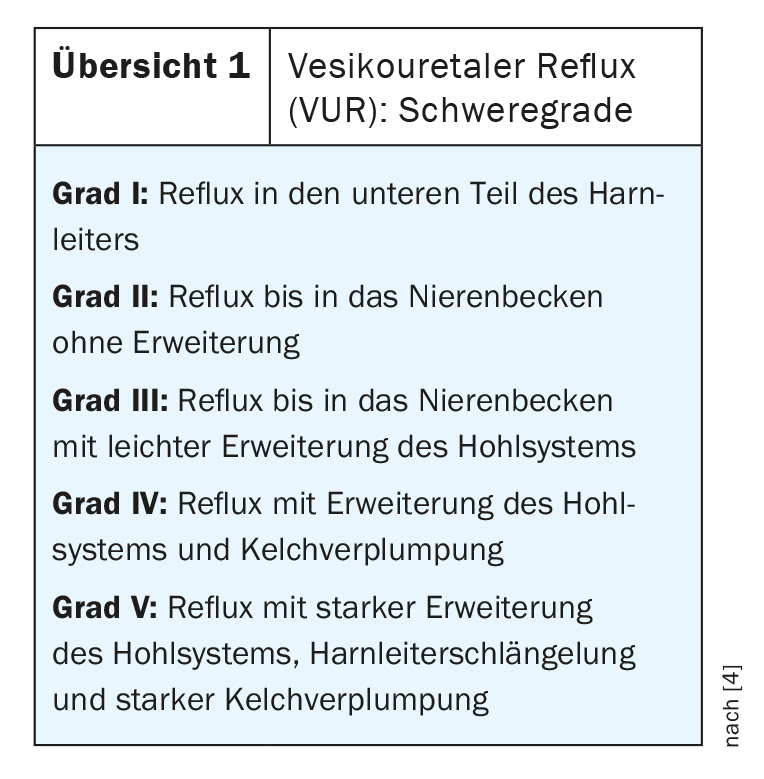
Vesicoureteral reflux (VUR), a urinary tract disorder divided into five degrees of severity ( Overview 1), affects approximately 1% of newborns. Usually, the valve mechanism of the ureteral orifice prevents urine from flowing back into the ureter and kidney. In VUR patients, this mechanism is disturbed. The refluxing urine may contain bacteria, leading to urinary tract infections (UTIs) or febrile urinary tract infections (fHWIs). Kidney infections and kidney damage can result. Therapy with Deflux® effectively stops the reflux of urine into the ureter. In studies, treatment proved successful in up to 93% of children with a VUR grade of 2-4 [2].
Advantageous safety profile
The use of Deflux® in children with VUR is minimally invasive. Using a cystoscope, the doctor injects the gel at the point where the ureter joins the bladder (also possible as a unilateral treatment). The injection stops the backflow of urine into the ureter and kidneys. Connective tissue cells subsequently infiltrate the injected gel and form new collagen tissue. In total, the procedure, which can be performed under general anesthesia, takes 15 minutes. The children can usually go about their usual activities the following day. The safety of Deflux® is contributed by microspheres made of dextranomer, which prevent migration of the gel due to their large format (80-250 μm) [3]. The hyaluronic acid contained is of non-animal origin and designed for optimal biocompatibility. The active ingredient has already been used in more than 40 million treatments worldwide [1].
Source: ARCO-Pharma
Literature:
- “Established therapy option complements urology specialist’s portfolio,” Farco-Pharma, Cologne, Feb. 17, 2021.
- Kalisvaart JF: Intermediate to long-term follow-up indicated low risk of recurrence after double hit endoscopic treatment for primary vesicoureteral reflux. J Ped Urol 2012; 8(4): 359-365.
- Deflux for the treatment of children with vesicoureteral reflux (VUR): Product monograph. Q-Med, AB. Uppsala, Sweden. 2005.
- Uni Tübingen: Vesicoureteral Reflux, www.medizin.uni-tuebingen.de (last accessed 03/16/2021).
HAUSARZT PRAXIS 2021; 16(4): 25