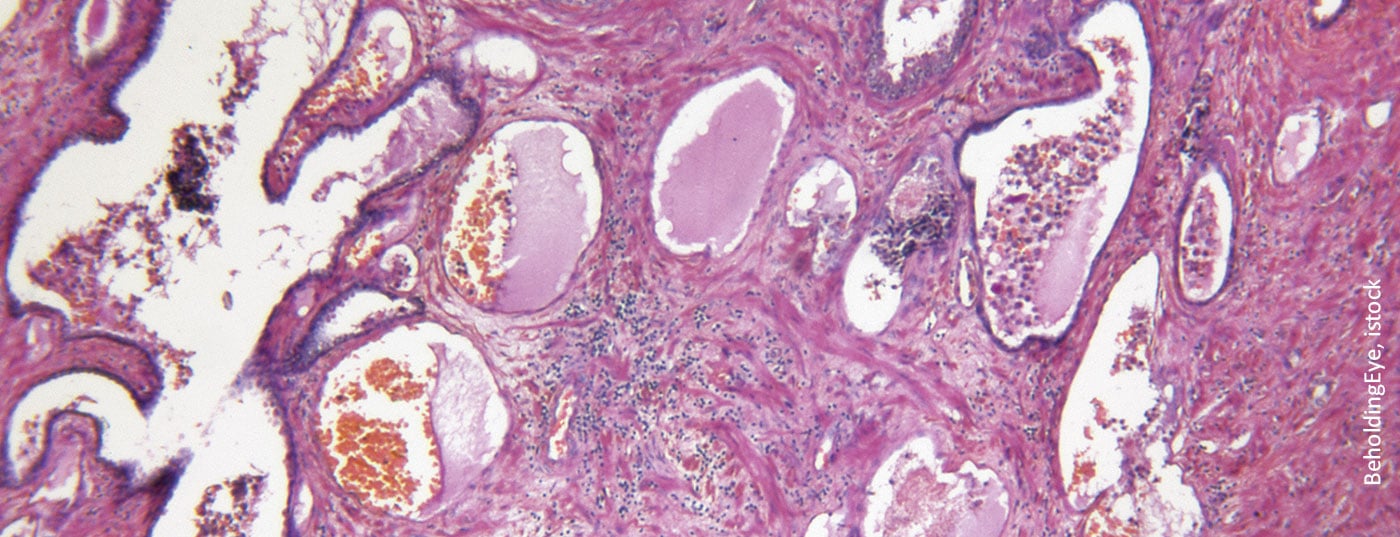
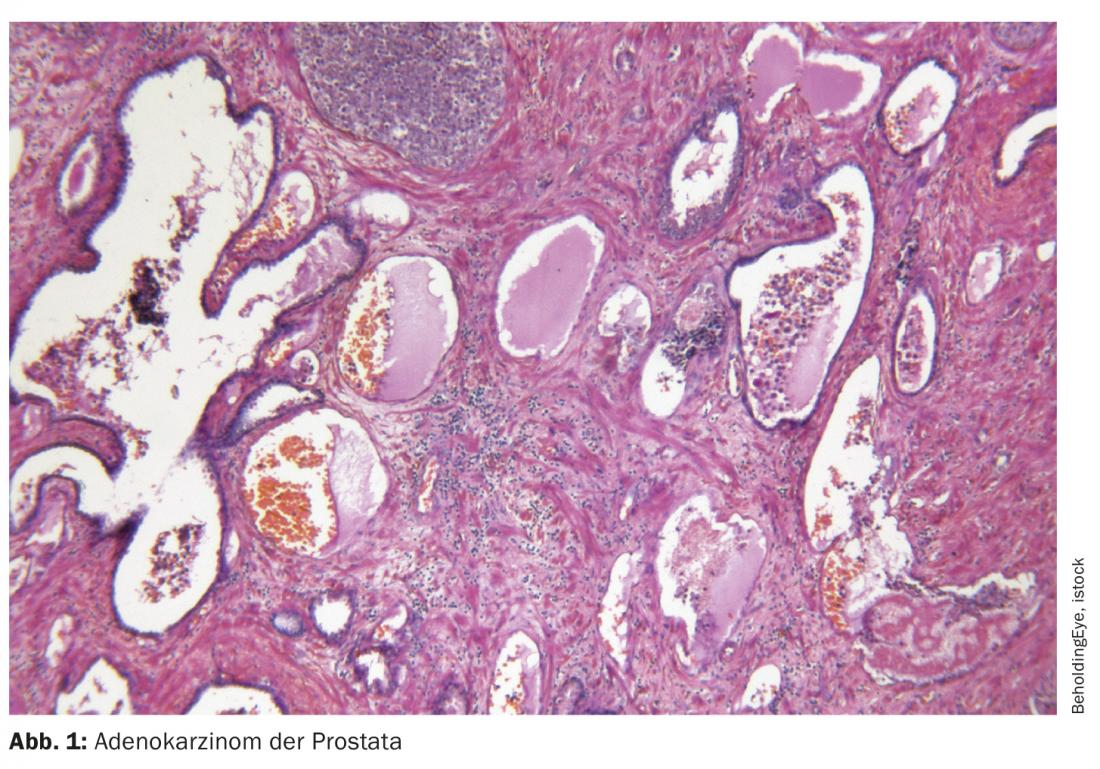
The prostate specific membrane antigen (PSMA) plays an increasingly important role not only in the diagnosis of prostate carcinoma. The radioligand 117Lutetium-PSMA-617also provides a new therapeutic option in advanced castration-resistant prostate cancer (mCRPC). This could soon advance into earlier stages of disease and lines of therapy.
Radiotherapy using 117Lutetium-PSMA-617was of great interest at all major oncology congresses this year. Reason enough for it to have its own session dedicated to it at the Advanced Prostate Cancer Consensus Conference (APCCC), which took place on October 9. In addition to current study results, clinical challenges and future hopes were discussed on this occasion – both in PSMA-based therapy and diagnostics.
PSMA: Small multiplication tables
The prostate specific membrane antigen (PSMA) is a transmembrane protein with the enzymatic function of a folate hydrolase carboxypeptidase. It has a short cytoplasmic domain, a long extracellular domain, and a hydrophobic transmembrane region [1,2]. PSMA is particularly – but not exclusively – expressed on epithelial prostate cells. In prostate cancer, up to 1000-fold overexpression is found compared to other tissue types. Most likely, the protein serves folic acid and glutamate uptake, but its exact role is still unclear. In addition to the prostate, PSMA is physiologically found in the lacrimal and salivary glands and in the small intestine. Slightly lower expression is detectable in liver, spleen, and parasympathetic ganglia, which can sometimes lead to false-positive results in PSMA-based imaging [3]. In addition, since the tracers used are excreted renally, uptake into the kidneys, ureters and bladder is unavoidable.
Pronounced intra- and interindividual differences in PSMA expression exist, which are of great importance in the clinic. On the one hand, without PSMA, the therapeutic target is missing; on the other hand, PSMA-negative lesions cannot be detected in PSMA-based imaging. Up to 10% of patients show no or minimal expression. In general, this is higher in mCRPC than in castration-sensitive prostate cancer (CSPC). Higher PSMA levels are associated with higher Gleason score and poorer overall survival [4]. Often, some foci in a patient express PSMA, while others remain invisible on PSMA PET – a clinical challenge.
As a potential therapeutic and diagnostic target, PSMA was recognized more than 30 years ago. The first FDA approval of a monoclonal antibody directed against PSMA followed as early as 1996: ProstaScint®. However, this antibody proved to be unreliable in clinical trials because it binds to the cytoplasmic domain of PSMA and therefore only colonizes apoptotic cancer cells. Recent approaches targeting PSMA are therefore based on attacking the extracellular domain of the transmembrane protein.
Of interest is the interaction of the androgen receptor with PSMA. Thus, androgen deprivation therapy (ADT) in both castration-sensitive and castration-resistant cases usually results in increased expression of PSMA [5,6]. Some metastases do not even appear on PSMA imaging until after ADT. Based on tumor response, castration-sensitive prostate cancer (CSPC) results in opposite effects of ADT on PSMA imaging: tumor cell numbers decrease, but these cells show higher PSMA expression.
PSMA in imaging
Meanwhile, PSMA-PET represents a robust imaging method with well-defined process and good global availability. Corresponding guidelines have existed since 2017, and an update is expected in 2022 [7]. Nevertheless, optimal use is not conclusively understood and some clinical challenges exist. Among others, there are a large number of tracers that have been insufficiently compared to date (overview 1) . Some comparisons with a few patients show similar results for the different substances [8,9]. The most robust data exist for the comparison between 18F-PSMA-1007and 68Ga-PSMA-11, presumably because these tracers are the most widely used. In this setting, 18F-PSMA-1007has been shown to yield more false positive results. In particular, bone lesions cannot be reliably detected with 18F-PSMA-1007due to physiological accumulation in the bone marrow. So, especially with this tracer, additional imaging such as MRI or CT should be used to interpret the images. On the other hand, 18F-PSMA-1007has been extremely useful in assessing locoregional conditions [10]. In terms of sensitivity, there seems to be no disadvantage of a tracer [11]. The bottom line is that different tracers are probably more appropriate depending on the setting – a field where there is still a lot to explore.
In addition to the lack of accurate characterization of various tracers, PSMA-PET also presents several other challenges, not the least of which is financial. Regardless of the substance used, the sensitivity of the method is never 100%. In addition, reliable assessment of the images requires a certain amount of experience – experience that cannot be taken for granted in view of the novelty. PSMA is by no means cancer, prostate or even prostate cancer specific. Expression is also found, for example, in lymphoma, lung carcinoma, and adenocarcinoma of the colon, as well as in various active inflammatory conditions such as Crohn’s disease or COVID-19. The clinical value of PSMA-based imaging and its impact on clinical practice also remain to be clarified in the future.
Thus, the red-hot question is whether PSMA-PET is indeed the best modality for reliable staging. This is a difficult question to address, especially since almost all clinical trials to date have used conventional imaging to assess tumor stage and lack randomized comparisons. Replacing or supplementing classical imaging with PSMA-PET could have lasting and difficult-to-estimate effects on clinical management including therapy and thus outcome. If, for example, previously undetected metastases are detected by the method, there is not least the risk of overtreatment. On the other hand, there are probably patients who would benefit from a more accurate assessment of their disease stage and thus from an intensification of treatment. The goal must remain to use imaging to identify those patients who really need an increase in intensity and thus improve their clinical outcomes.
To illustrate this issue, some impressive numbers were presented at APCCC. Thus, 98% of CRPC patients who are considered M0 in conventional staging have lesions in PSMA-PET, of which again 24% are local and 76% are N1/M1 [12]. Overall, about half of the original M0 patients thus become M1 patients – an upstaging with consequences. Because even though the treatment options are similar in metastatic and non-metastatic CRPC, according to the current approval status, the option of apalutamide or darolutamide is lost [13]. With new modalities in imaging, clinical management must always be questioned and adapted – a process that is currently in full swing in prostate cancer. That PSMA-PET is superior to conventional CT and choline PET, which was in use until 2016, in terms of specificity and sensitivity is beyond question. However, the clinical value remains to be clarified, optimally in the context of randomized clinical trials.
PSMA-based radiotherapy
As controversial as the use of PSMA-PET may be in staging, it is clear in patient selection for therapy using PSMA-directed radioligands. As such, 117Lutetium-PSMA-617is already accessible at specialized centers in Switzerland under a compassionate use program, although Swissmedic approval is not yet available [13]. To date, treatment is used after failure of an AR-targeting agent and a taxane (docetaxel) in mCRPC. The ideal therapeutic sequence with cabazitaxel is currently unclear and must be assessed on an individual basis. Due to the different nature of the therapy, it has a high potential to optimally complement existing options. Moreover, with an expression rate of approximately 87% in mCRPC, PSMA represents a nearly ubiquitous target including biomarker for response. PSMA-targeted treatment could thus be an option for a large number of affected individuals in the future – and the identification of suitable patients is not particularly complicated.
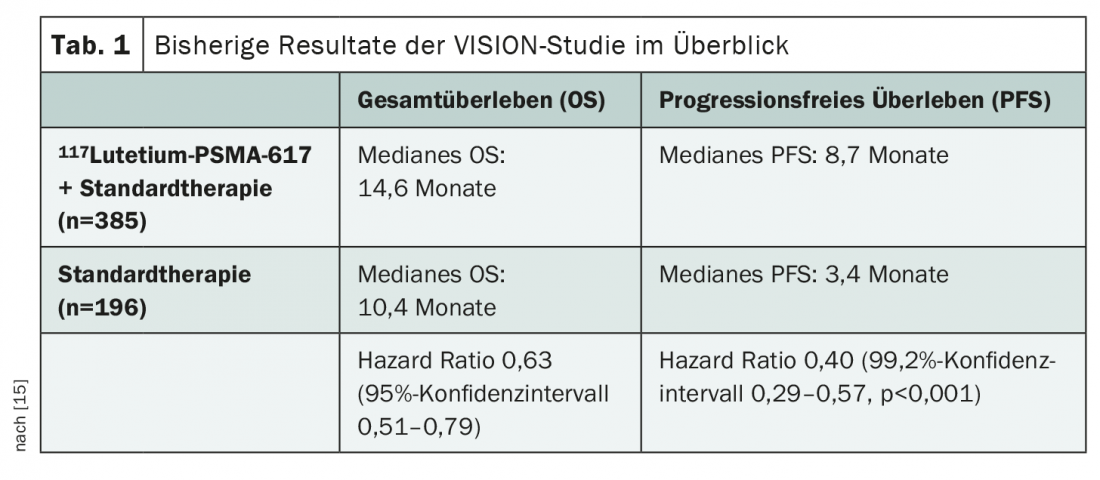
Most of the data available to date on 117Lutetium-PSMA-617comes from the recently published VISION study presented at various congresses [14,15]. This investigates the addition of 117Lutetium-PSMA-617to standard therapy in mCRPC after pretreatment with at least one androgen receptor blocker and a taxane-based chemotherapy regimen in the setting of positive 68Gallium-PSMA-11 PET/CT. Significant benefits in overall survival (OS) and progression-free survival (PFS) across all subgroups have already been demonstrated (Table 1) . Given the demonstrated efficacy of 117Lutetium-PSMA-617 therapyin this setting, various further questions now arise. For example, the optimal timing for treatment still needs to be clarified. Should 117Lutetium-PSMA-617be used before, with, or after chemotherapy? And could radioligand treatment be used in earlier stages of disease, such as CSPC or non-metastatic cases? What is the situation regarding subsequent treatment options? Reliable data for the assessment of long-term toxicity, in particular bone marrow suppression, are also lacking to date. This issue is likely to become increasingly prominent with testing in earlier and earlier stages of disease and lines of therapy.
Some studies are currently investigating the optimal treatment sequence using 117Lutetium-PSMA-617. For example, the PSMAfore trial is ongoing, testing the efficacy of radioligand in mCRPC after an androgen receptor blocker but before chemotherapy. The PSMAddition trial, which is testing the addition of 117Lutetium-PSMA-617to standard treatment in first-line mCSPC, is taking an even earlier application under the microscope. Even in the neoadjuvant setting, a trial is currently underway: LuTectomy. While the benefit in mCRPC after taxane-based chemotherapy is now considered established, its use in earlier lines of therapy and stages of disease will be seen over the next several years.
Patient selection as the key?
The fact that 117Lutetium-PSMA-617 therapyonly resulted in a PSA decrease of <50% in most patients in the VISION study was attributed by the experts at the APCCC, among other things, to the inadequate patient selection to date. It is likely that not all included patients benefited from the additional treatment. This may indicate that other patient selection methods should be used in addition to PSMA-PET to achieve optimal clinical efficacy. Such could be genetic in nature, but additional FDG-PET is also an option.
The genetic profile in prostate cancer is extremely heterogeneous, for example, de novo metastatic disease often shows a less favorable genetic profile with poorer outcome [16]. These patients respond less well to a number of therapies, which may also be true for 117Lutetium-PSMA-617 treatment. However, there is a lack of reliable data on this – a reason to determine the genetic profile within the framework of clinical studies. Thus, in the future, in addition to PSMA expression, the genetic profile could also be used in treatment decisions.
It is also unclear whether FDG-PET should be used for patient selection in addition to PSMA-. One was not performed in the VISION trial [14] – unlike in the TheraP trial, which compared treatment using 117Lutetium-PSMA-617with cabazitaxel in mCRPC [17]. The additional FDG-PET led on the one hand to more study exclusions – and on the other hand to a higher response rate to 117Lutetium-PSMA-617 treatment of66% (VISION: 46%). This may be due to the fact that those patients who had PSMA-expressing metastases as well as those without corresponding expression could be identified and excluded thanks to FDG-PET. Apart from a potential benefit in patient selection, performing FDG-PET also brings advantages in more accurate characterization of the disease and optimization of biopsy sampling sites. For example, symptoms caused by metastases can be better assigned and properly treated at an early stage. In general, the often present intraindividual heterogeneity of metastatic prostate carcinoma is an important limitation for treatment by radioligands. This can be detected by additional FDG-PET.
Dreams of the future: Of combinations and new active ingredients
The development of 117Lutetium-PSMA-617lays the foundation for a new therapeutic option in prostate cancer. These will have to be examined more closely in the coming years and characterized in terms of their potential. Optimal therapeutic combinations as well as sequences still need to be found. In addition, alternative treatment approaches exist that also target PSMA.
A broad field of research is opening up in the area of possible combination therapies with 117Lutetium-PSMA-617. Appropriate drug combinations could, among other things, prevent resistance due to the heterogeneity of the disease. Combinations with enzalutamide in the first-line setting (ENZA-P), and olaparib (LuPARP) and pembrolizumab (PRINCE) in the second-line setting are currently being investigated in mCRPC. And things are also changing with regard to possible subsequent therapies. Here, the focus is particularly on α-emitters. On the one hand, these could be used after the β-emitter 117Lutetium-PSMA-617, and on the other hand, they could be a more effective and gentler alternative to it. This is because α-emitters cause a greater energy transfer at a shorter range. Currently, the compounds 225Act-PSMA-617, 225Act-J591and 227Thorium-PSMA-TTCare being investigated in Phase I trials.
Alternative drug classes targeting PSMA include CAR-T cells and bispecific antibodies (BiTE, Bispecific T-Cell Engagers) such as Acapatamab. Currently, these are still in their infancy and development is also proving difficult given the advanced stage of the disease with high vulnerability of the patient population. Nevertheless, first hopeful results from Phase I trials exist with response rates around 34% [18]. We remain curious.
Source: PSMA in diagnostics and therapy. Session 2, Advanced Prostate Cancer Consensus Conference (APCCC) online, Oct 09, 2021.
Literature:
- Rajasekaran AK, Anilkumar G, Christiansen JJ: Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005; 288(5): C975-981.
- Sengupta S, et al: Comparison of prostate-specific membrane antigen ligands in clinical translation research for diagnosis of prostate cancer. Cancer Rep (Hoboken). 2019; 2(4): e1169.
- Hofman MS, et al: Prostate-specific membrane antigen PET: Clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018; 38(1): 200-217.
- Paschalis A, et al: Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019; 76(4): 469-478.
- Bakht MK, et al: Influence of Androgen Deprivation Therapy on the Uptake of PSMA-Targeted Agents: Emerging Opportunities and Challenges. Nucl Med Mol Imaging. 2017; 51(3): 202-211.
- Hope TA, et al: 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J Nucl Med. 2017; 58(1): 81-4.
- Fendler WP, et al: (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017; 44(6): 1014-1024.
- Giesel FL, et al: Intraindividual Comparison of (18)F-PSMA-1007 and (18)F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. J Nucl Med. 2018; 59(7): 1076-80.
- Dietlein M, et al: Comparison of [(18)F]DCFPyL and [ (68)Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Mol Imaging Biol. 2015; 17(4): 575-584.
- Dietlein F, et al: Intraindividual Comparison of (18)F-PSMA-1007 with Renally Excreted PSMA Ligands for PSMA PET Imaging in Patients with Relapsed Prostate Cancer. J Nucl Med. 2020; 61(5): 729-734.
- Rauscher I, et al: Matched-pair Comparison of (68)Ga-PSMA-11 PET/CT and (18)F-PSMA-1007 PET/CT: Frequency of Pitfalls and Detection Efficacy in Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2020; 61(1): 51-57.
- Fendler WP, et al: Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2019; 25(24): 7448-7454.
- Swissmedic drug information: www.swissmedicinfo.ch (last accessed 10/21/2021).
- Sartor O, et al: Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12): 1091-1103.
- Morris MJ, et al: Phase III study of lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION). ASCO Annual Meeting 2021, Plenary Session Genitourinary Cancer – Prostate, Testicular, and Penile, Abstract #LBA4.
- Deek MP, et al: The Mutational Landscape of Metastatic Castration-sensitive Prostate Cancer: The Spectrum Theory Revisited. Eur Urol. 2021; 80(5): 632-640.
- Hofman MS, et al: [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021; 397(10276): 797-804.
- Einsele H, et al.: The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer. 2020; 126(14): 3192-3201.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(6): 26-28 (published 8/12/21, ahead of print).