Several risk factors have now been identified which predispose to the development of joint involvement in the presence of psoriasis. Dermatologists play a key role in early detection. Adequate systemic therapy in a “window of opportunity” can have a positive impact on long-term outcomes. Although the treatment options have improved, the response to therapy is not satisfactory for all PsA patients. Research is therefore continuing at a feverish pace and new treatment strategies are being tested.
Minimal disease activity ( MDA) is considered a successful therapy for psoriatic arthritis (PsA). Progress in deciphering the pathogenetic relationships of PsA has driven the development of modern system therapeutics, with the result that biologics and “small molecules” are now available that are directed against various targets: TNF, interleukin (IL)-12/23, IL-17, IL-23, JAK-STAT, PDE-4. The therapy results have been raised to a new level (Tab. 1) [1]. While conventional synthetic disease-modifying antirheumatic drugs (csDMARDs**) achieved an MDA in 17% of PsA patients according to a systematic review published in 2020, treatment with biologics (bDMARDs) achieved an MDA in 57% [2]. Although this is a quantum leap, it also reflects a significant proportion of non-responders.
** csDMARDs: in particular methotrexate, also sulfasalazine or leflunomide; in contrast to targeted synthetic (tsDMARDs) such as apremilast and biologics (bDMARDs)
GRAPPA and EULAR recommendations
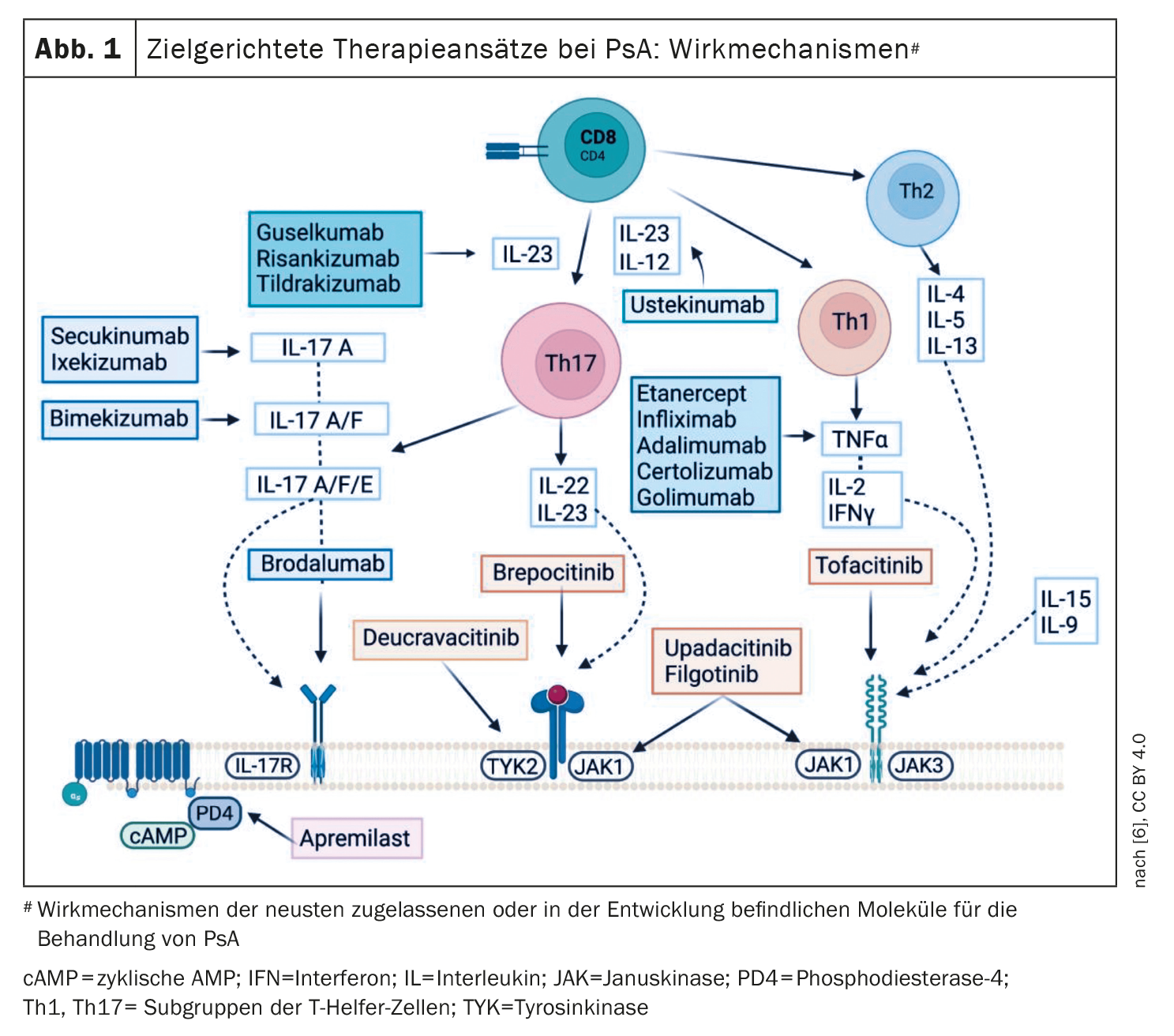
In view of the heterogeneity of PsA, a personalized treatment strategy is becoming increasingly important. The current recommendations of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR) suggest addressing all active disease domains and PsA-relevant comorbidities of a given patient [3,4]. The current S3 psoriasis guideline recommends that dermatologists should carry out the diagnosis and treatment selection for PsA on an interdisciplinary basis in cooperation with a rheumatologist [5]. According to the EULAR and GRAPPA treatment algorithm, csDMARDs are considered first-line treatment, followed by the PDE-4 inhibitor apremilast, biologics (bDMARDs) or the use of targeted synthetic DMARDs such as Janus kinase (JAK) inhibitors [3,4]. The DMARDs currently approved in Switzerland in the indication area of psoriatic arthritis are shown in Figure 1.
Apremilast and TNF-α-i are tried and tested treatment options
Apremilast is an oral small molecule that inhibits phosphodiesterase 4 (PDE-4). PDE-4 inhibition promotes an increase in intracellular cyclic AMP, which prevents the synthesis of proinflammatory cytokines and increases anti-inflammatory cytokines (IL-10) [6]. Guidelines recommend apremilast in particular for psoriasis with nail involvement, peripheral PsA, enthesitis and dactylitis. The ESTEEM studies demonstrated efficacy in plaque psoriasis and nail involvement and the PALACE studies provided evidence of efficacy in PsA [7,8]. In addition, in several randomized controlled trials (RTC), an ACR20 response was achieved significantly more frequently at week 16 with both apremilast doses (20 mg or 30 mg, 2x/d) than with placebo [9–12].
TNF-α inhibitors are a proven biological treatment option for PsA, for which there is evidence of efficacy for all PsA domains [6]. According to a meta-analysis, adalimumab, etanercept and infliximab are equivalent in terms of ACR20 response [13].
“Hit hard and early” with highly effective biologics?
One approach to optimizing treatment outcomes is to intervene as early as possible with highly effective biologics so that the development of PsA can be delayed or even prevented [14]. Psoriasis patients with a high risk of developing PsA (arthralgia, nail or head psoriasis or PASI>6) received treatment with the IL-17A inhibitor secukinumab in the IVEPSA study. After a treatment period of 24 weeks, skin lesions as well as arthralgia and synovitis scores (evaluated by CT and MRI) had improved [15]. And in another study, the IL12/23 inhibitor ustekinumab led to a reduction in peripheral subclinical enthesopathy in 23 patients with moderate to severe plaque psoriasis after 12 weeks, with this effect lasting until week 52 [16]. And there are also study findings on the IL-23 inhibitor guselkumab that point in a similar direction [17].
Head-to-head studies on IL-17A inhibitors
Over the last decades, it has become clear that Th17 cells, IL-23 and IL-17 play a central immunopathological role [1]. As a result, TNF-α antagonists are no longer the first choice among biologics for PsA; instead, ustekinumab and IL-17A inhibitors are gaining ground. In PsA patients who had responded inadequately to csDMARDs, IL-17A-i ixekizumab proved to be superior to adalimumab in a head-to-head study, both in terms of improvement in enthesitis and skin lesions [18]. And in a head-to-head study by McInnes et al. Secukinumab was at least as effective as the TNF-α inhibitor for the musculoskeletal endpoints, but performed better in terms of improvement in skin lesions [19]. According to Sundanum et al. 2023, which show that IL-17A-i in PsA has a growing evidence base in terms of efficacy and safety [1]. The IL-17A/F inhibitor bimekizumab is currently only approved for plaque psoriasis in Switzerland, but in the BE COMPLETE study it proved to be superior to placebo in terms of ACR50 response in patients who had previously received TNF-α-i therapy [20].
IL-23-i – two representatives approved for PsA
Risankizumab and guselkumab are currently approved in the PsA indication area in Switzerland. In the KEEPsAKE studies, significantly more patients treated with risankizumab achieved an ACR-20 response at week 24 compared to placebo [21,22]. The extension of the indication for guselkumab is based on the DISCOVER studies. Both treatment-naïve patients and those previously treated with TNF-α-i showed a significantly higher ACR20 response with guselkumab at week 24 compared to placebo [23–25]. Tildrakizumab, another IL-23-i approved for plaque psoriasis, has positive results from a phase II study in PsA; the phase III INSPIRE study program has not yet been completed [26–28].
And the JAK/STAT signaling pathway?
The JAK family consists of four members: Janus kinase (JAK)-1, JAK-2, JAK-3 and tyrosine kinase (TYK)-2. Immunomodulatory and proinflammatory effects are mediated via the JAK/STAT signaling pathway. Tofacitinib specifically inhibits JAK1 and JAK3. A phase III study demonstrated the efficacy of tofacitinib compared to placebo in both treatment-naïve PsA patients and in patients after TNF-α-i treatment failure [30,31]. Upadacitinib inhibits JAK1 and was shown to be superior to placebo in the Phase III SELECT-PsA 1 study in terms of ACR20 response. In addition, upadacitinib 15 mg proved to be non-inferior to adalimumab, while the JAK-i at the 30 mg dose was even superior to adalimumab, although more serious adverse events occurred with upadacitinib [32]. And in SELECT-PsA, PsA patients in whom TNF-α-i was not effective or who could not tolerate it achieved a significantly higher ACR20 response and a higher MDA rate with upadacitinib (15 mg or 30 mg per day) [33]. In a phase II study, deucravacitinib, a TYK2 inhibitor, was also shown to be superior to placebo at both doses (6 mg and 12 mg/day) in arthritis, enthesitis and dactylitis [34].
Regarding the safety of members of the JAK family, the ORAL surveillance study found that patients with rheumatoid arthritis treated with tofacitinib had a higher risk of cardiovascular events than those treated with TNF-α-i [35]. As a result, both the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have issued a warning regarding the use of JAK-i in patients over 65, smokers and in the presence of cardiovascular risk factors, thromboembolic events or a history of malignant disease [6].
Literature:
- Sundanum S, Orr C, Veale D: Targeted Therapies in Psoriatic Arthritis-An Update. Int J Mol Sci 2023 Mar 28; 24(7):6384
- Zardin-Moraes M, et al: Prevalence of Psoriatic Arthritis Patients Achieving Minimal Disease Activity in Real-world Studies and Randomized Clinical Trials: Systematic Review with Metaanalysis. J Rheumatol 2020; 47: 839.
- Gossec L, et al: EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020; 79: S700-S712.
- Coates LC, et al: Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022; 18: 465-479.
- Nast A et al. German S3 guideline on the treatment of psoriasis vulgaris, adapted by EuroGuiDerm, register.awmf.org/assets/guidelines/013-001l_S3_Therapie-Psoriasis-vulgaris_2021-07-verlaengert.pdf, (last accessed 23.01.2024)
- Azuaga AB, Ramírez J, Cañete JD: Psoriatic Arthritis: Pathogenesis and Targeted Therapies. Int J Mol Sci 2023; 24(5): 4901. doi: 10.3390/ijms24054901. www.mdpi.com/1422-0067/24/5/4901#,(last accessed 23.01.2024)
- Papp K, et al: Apremilast, an Oral Phosphodiesterase 4 (PDE4) Inhibitor, in Patients with Moderate to Severe Plaque Psoriasis: Results of a Phase III, Randomized, Controlled Trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). JAAD 2015; 73: 37-49.
- Paul C, et al. Efficacy and Safety of Apremilast, an Oral Phosphodiesterase 4 Inhibitor, in Patients with Moderate-to-severe Plaque Psoriasis over 52 Weeks: A Phase III, Randomized Controlled Trial (ESTEEM 2). Br J Dermatol 2015; 173: 1387-1399.
- Kavanaugh A, et al: Extended Report: Treatment of Psoriatic Arthritis in a Phase 3 Randomized, Placebo-Controlled Trial with Apremilast, an Oral Phosphodiesterase 4 Inhibitor. Ann Rheum Dis 2014; 73: 1020.
- Cutolo M, et al: A Phase III, Randomized, Controlled Trial of Apremilast in Patients with Psoriatic Arthritis: Results of the PALACE 2 Trial. J Rheumatol 2016; 43: 1724-1734.
- Edwards CJ, et al: Extended Report: Apremilast, an Oral Phosphodiesterase 4 Inhibitor, in Patients with Psoriatic Arthritis and Current Skin Involvement: A Phase III, Randomized, Controlled Trial (PALACE 3). Ann Rheum Dis 2016; 75: 1065.
- Wells AF, et al: Apremilast Monotherapy in DMARD-Naive Psoriatic Arthritis Patients: Results of the Randomized, Placebo-Controlled PALACE 4 Trial. Rheumatology 2018; 57: 1253.
- Fénix-Caballero S, et al: Direct and Indirect Comparison of the Efficacy and Safety of Adalimumab, Etanercept, Infliximab and Golimumab in Psoriatic Arthritis. J Clin Pharm Ther 2013; 38: 286-293.
- Kimak A, et al: Psoriatic Arthritis: Development, Detection and Prevention: A Scoping Review. J Clin Med 2023; 12(11): 3850
- 15 Kampylafka E, et al. Disease interception with interleukin-17 inhibition in high-risk psoriasis patients with subclinical joint inflammation-data from the prospective IVEPSA study. Arthritis Res 2019; 21: 178.
- Savage L, et al: Regression of Peripheral Subclinical Enthesopathy in Therapy-Naive Patients Treated With Ustekinumab for Moderate-to-Severe Chronic Plaque Psoriasis: A Fifty-Two-Week, Prospective, Open-Label Feasibility Study. Arthritis Rheumatol 2019; 71 : 626-631.
- Haberman RH, et al: Efficacy of guselkumab, a selective IL-23 inhibitor, in Preventing Arthritis in a Multicentre Psoriasis At-Risk cohort (PAMPA): Protocol of a randomized, double-blind, placebo controlled multicentre trial. BMJ Open 2022; 12:e063650.
- Mease PJ, et al: A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomized, open-label, blinded-assessor trial. Ann Rheum Dis 2020;79: 123-131.
- McInnes IB, et al: Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): A double-blind, parallel-group, randomized, active-controlled, phase 3b trial. Lancet 2020; 395: 1496-1505.
- Merola JF, et al: Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumor necrosis factor-α inhibitors: A randomized, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023; 401: 38-48.
- Kristensen LE, et al: Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 24-Week Results from the Randomized, Double-Blind, Phase 3 KEEPsAKE 1 Trial. Ann Rheum Dis 2022; 81: 225-231.
- Östör A, et al: Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 24-Week Results from the Randomized, Double-Blind, Phase 3 KEEPsAKE 2 Trial. Ann Rheum Dis 2022; 81: 351-358.
- Deodhar A, et al: Guselkumab in Patients with Active Psoriatic Arthritis Who Were Biologic-Naive or Had Previously Received TNFα Inhibitor Treatment (DISCOVER-1): A Double-Blind, Randomized, Placebo-Controlled Phase 3 Trial. Lancet 2020: 395: 1115-1125.
- Mease PJ, et al: Guselkumab in Biologic-Naive Patients with Active Psoriatic Arthritis (DISCOVER-2): A Double-Blind, Randomized, Placebo-Controlled Phase 3 Trial. Lancet 2020; 395: 1126-1136.
- Coates LC, et al: Efficacy and Safety of Guselkumab in Patients with Active Psoriatic Arthritis Who Are Inadequate Responders to Tumor Necrosis Factor Inhibitors: Results through One Year of a Phase IIIb, Randomized, Controlled Study (COSMOS). Ann Rheum Dis 2022; 81: 359-369.
- ClinicalTrials.Gov. Identifier: NCT04314544, https://clinicaltrials.gov,(last retrieval 23.01.2024)
- ClinicalTrials.Gov. Identifier: NCT04314531, https://clinicaltrials.gov,(last retrieval 23.01.2024)
- Mease PJ, et al: Efficacy and Safety of Tildrakizumab in Patients with Active Psoriatic Arthritis: Results of a Randomized, Double-Blind, Placebo-Controlled, Multiple-Dose, 52-Week Phase IIb Study Ann Rheum Dis 2021; 80: 1147-1157.
- Swissmedic: Medicinal product information, https://swissmedicinfo.ch,(last accessed 23.01.2024)
- 30 Gladman D, et al: Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. NEJM 2017; 377: 1525-1536.
- Mease P, et al. Tofacitinib or Adalimumab versus Placebo for Psoriatic Arthritis. NEJM 2017; 377: 1537-1550.
- McInnes IB, et al: Upadacitinib in Patients with Psoriatic Arthritis and an Inadequate Response to Non-Biological Therapy: 56-Week Data from the Phase 3 SELECT-PsA 1 Study. RMD Open 2021; 7: e00183
- Mease PJ, et al: Upadacitinib for Psoriatic Arthritis Refractory to Biologics: SELECT-PsA 2 Ann Rheum Dis 2021; 80: 312-320.
- Mease PJ, et al: Efficacy and Safety of Selective TYK2 Inhibitor, Deucravacitinib, in a Phase II Trial in Psoriatic Arthritis. Ann Rheum Dis 2022; 81: 815-822.
- Ytterberg SR, et al: Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. NEJM 2022; 386: 316-326.
DERMATOLOGY PRACTICE 2024; 34(1): 40-41