Chronic destruction of red blood cells is characteristic of paroxysmal nocturnal hemoglobinuria. Mutations in the PIG-A gene lead to a GPI-AP deficit that results in uncontrolled complement activation. C5 complement inhibition, which only needs to be administered every eight weeks for the first time, may help.
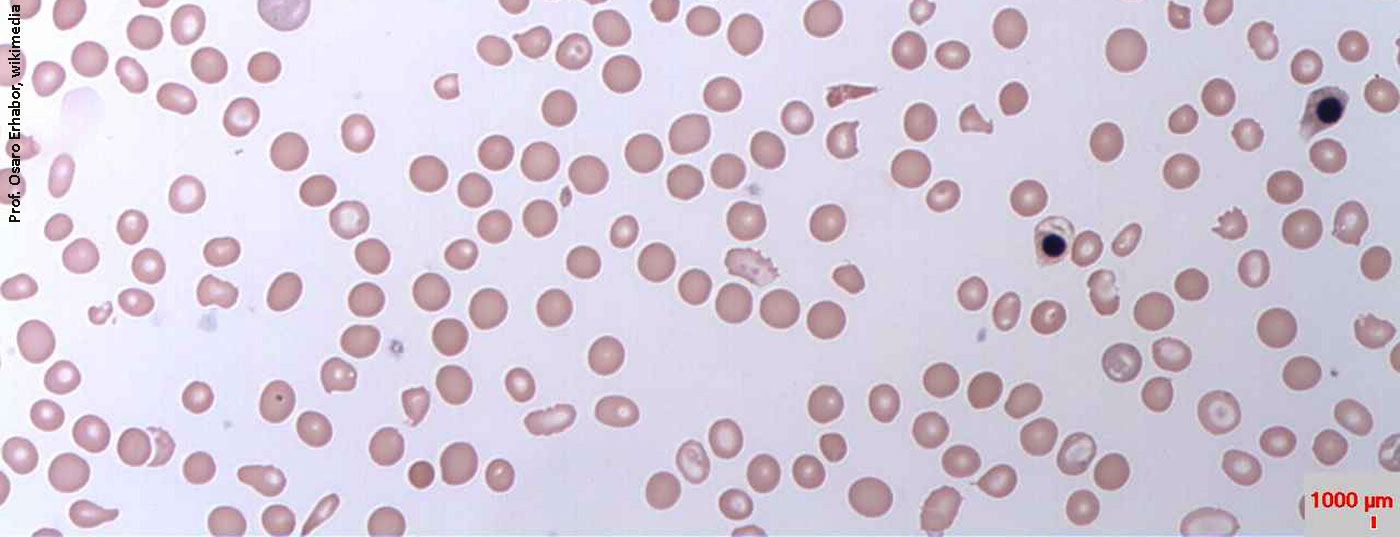
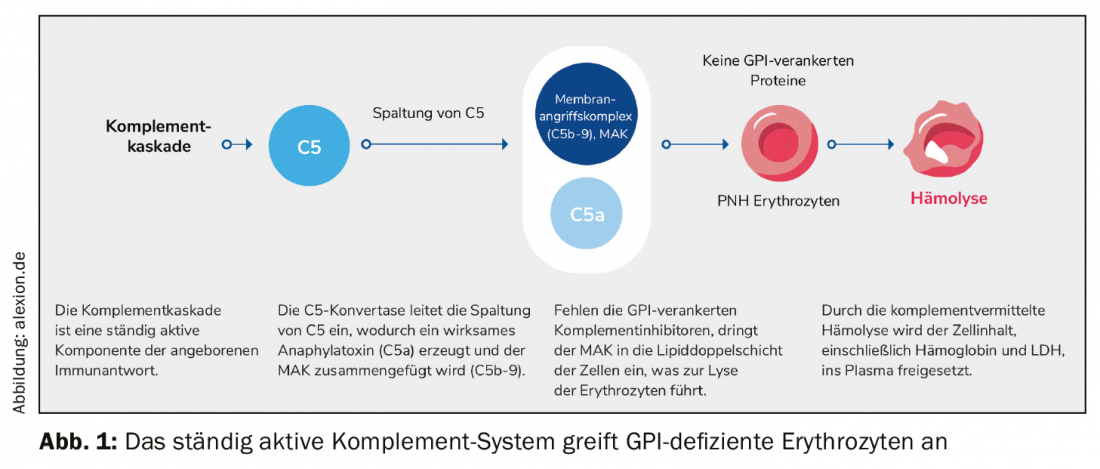
The main feature of rare, chronic and progressive paroxysmal nocturnal hemoglobinuria (PNH) is complement-mediated hemolysis. Due to an acquired mutation, glycophosphatidylinostiol (GPI) anchor proteins are absent in bone marrow hematopoietic stem cells. As a result, GPI-anchored proteins cannot be formed, especially on the surface of red erythrocytes. Protection from the complement system, part of the body’s immune system, is eliminated. The red blood cells are mistaken for invaders, attacked and destroyed. The membrane attack complex (MAK) penetrates the lipid bilayer of the cell and triggers lysis of the erythrocytes there. The entire cell content, including hemoglobin and LDH, is released into the plasma (Fig. 1).
Complex disease with variable symptoms
Characteristics of the disease include hemolytic anemia, hemoglobinuria, and bone marrow insufficiency or failure. It is classically manifested mainly by fatigue, a reduced quality of life and dyspnea on exertion. However, nonspecific symptoms often associated with hemolytic crises may also occur. However, the main cause of increased patient morbidity and mortality is thrombophilia. Up to 50% of all affected individuals develop thrombosis without specific therapeutic measures. 35% of patients die within 5 years of diagnosis.
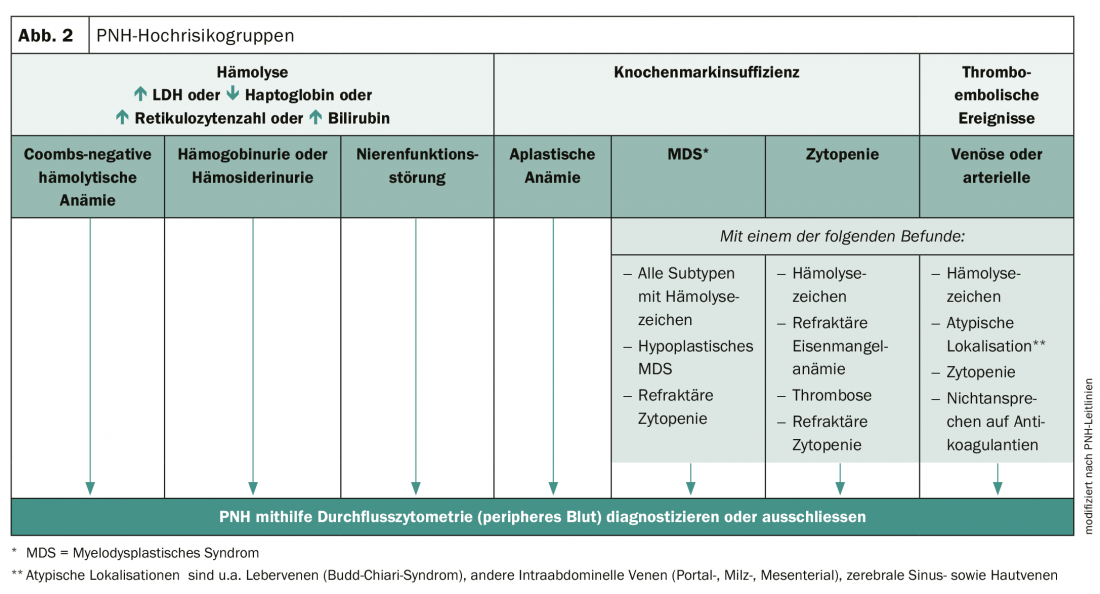
Early diagnosis is essential to improve the prognosis of those affected. PNH can be diagnosed using high-sensitivity flow cytometry and a comprehensive clinical examination. However, many physicians are unaware of the complexity of symptoms and variability of the disease. As a result, PNH is often not recognized and diagnosis is delayed from one to more than 5 years. However, there are high-risk groups in whom the presence of PNH should be considered (Fig. 2).
Therapy management improves
To halt hemolysis, complement inhibitor is used to interfere with the terminal functions of the complement cascade. Proximal functions, on the other hand, remain. For example, the rate of thromboembolic events (TE) in anticoagulant-treated patients was significantly reduced from 11.54 to 0.72. The inhibitor also showed convincing long-term clinical benefits. The only drawback: the short infusion interval. Now the inhibitor could be further developed and the half-life extended (Ravulizumab). The reduction to 6-7 infusions per year significantly improves the quality of life of those affected and also reduces the risk of breakthrough hemolysis from 10.7% to 4%.
Source: Annual Meeting 2019 of the German-Speaking Societies of Hematology and Medical Oncology (DGHO)
InFo ONCOLOGY & HEMATOLOGY 2019; 7(6): 32-33 (published 12/6/19, ahead of print).