Patients with chronic inflammatory rheumatic diseases often suffer from pain, comorbidities, and physical and social limitations, resulting in immense impairment of quality of life [1, 2]. In order to improve the situation of those affected by RA, AS and PsA, the achievement of remission is the primary therapeutic goal [1, 3, 4].
In Switzerland, approximately 85000 individuals are affected by rheumatoid arthritis (RA) and 70000 by ankylosing spondyloarthritis (AS; ankylosing spondylitis), of the latter probably only 10000 are correctly diagnosed [5, 6]. Psoriatic arthritis (PsA) occurs in up to 15% of all psoriasis patients and affects between 24000 and 80000 people in Switzerland, depending on the study [7, 8]. In addition to the inflammation of the musculoskeletal system and the great suffering of those affected, the three chronic inflammatory rheumatic diseases have in common a heterogeneous clinical picture that requires personalized therapeutic management [3, 4, 9].
When is remission achieved?
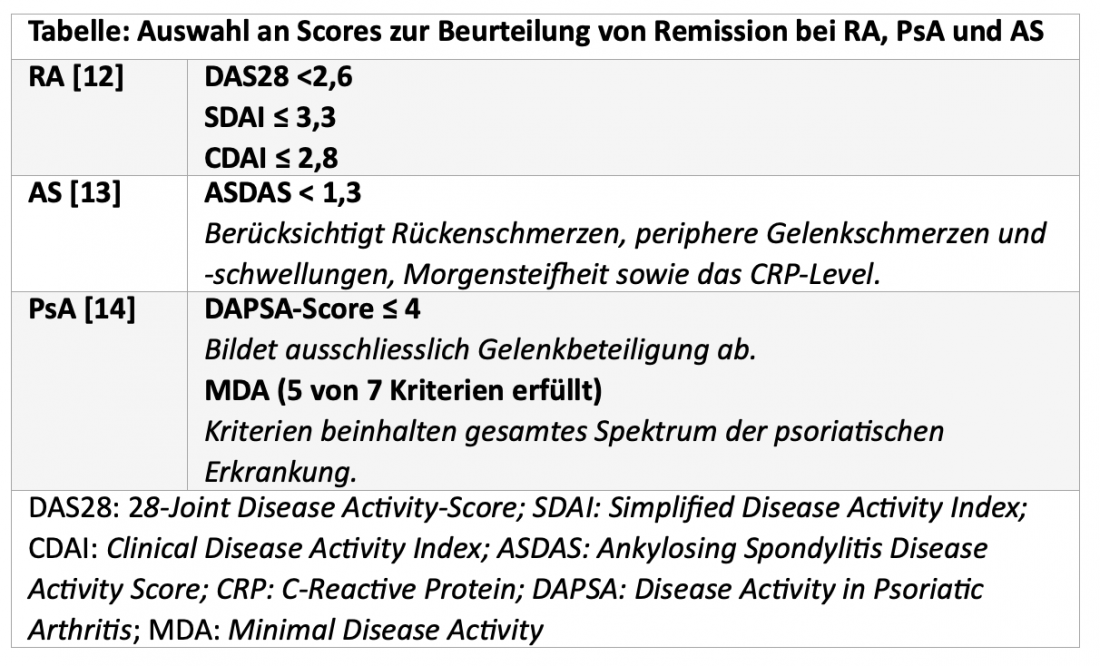
The ultimate goal is to achieve remission, a state in which the disease is completely inactive or so inactive that the patient no longer notices it. In this context, early diagnosis and a correspondingly early start of treatment are associated with an improved prognosis [1, 3, 4, 10, 11]. To assess whether remission has been achieved, various scores are used in clinical trials (Table).
According to the current EULAR treatment recommendations for the management of RA, disease activity should be monitored regularly and therapy should be adjusted if no improvements are observed after three months of treatment or if the treatment goal is not reached after six months [4]. And in AS and PsA, it is also recommended that treatment be adjusted in the absence of a desired response, although the time window for evaluating treatment success is not explicitly addressed here [3, 9].
Systemic treatment approaches with different mechanisms of action
In Switzerland, several systemic approaches with different mechanisms of action are available for the treatment of patients with RA, PsA and AS. In addition to tumor necrosis factor inhibitors (TNFi), the selective Janus kinase inhibitor (JAKi) upadacitinib (UPA; RINVOQ®) can also be used in all three indications [15, 16]. The two treatment options differ in several respects:
- TNFi inhibit a specific cytokine, JAKi affect many immunological signaling pathways and thus have a broad spectrum of activity [17].
- UPA has a much shorter half-life of 9 to 14 hours than the TNFi adalimumab (ADA) of about 2 weeks and is metabolized more rapidly, which may be useful, for example, during surgery [15, 16].
- UPA is taken 1x daily as a tablet, making therapy more convenient and uncomplicated for many patients than biweekly injections of ADA [15, 16].
The efficacy and safety of UPA in RA, AS, and PsA have been evaluated in the comprehensive SELECT program in 11 studies with a total of more than 8000 patients [18-28].
UPA in RA – remission rates of >40% after 26 weeks. [20]
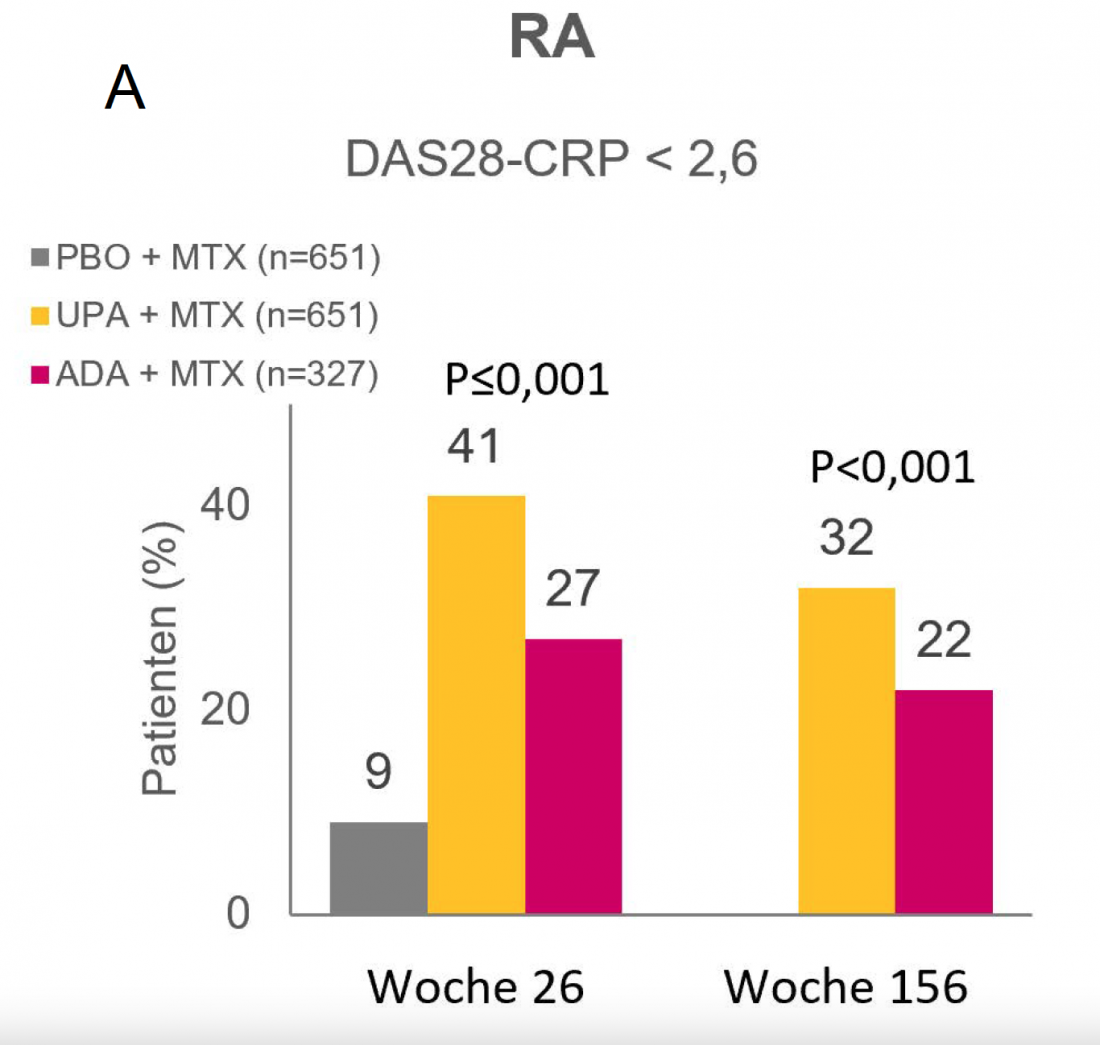
In the randomized phase III SELECT-COMPARE trial, 41% of patients with RA who had previously responded inadequately to methotrexate (MTX) achieved DAS28-CRP < 2.6 remission after 26 weeks on UPA + MTX. This was achieved by 27% under ADA + MTX and 9% under PBO + MTX (both P<0.001) (Fig. 1A) [20]. Patients who completed the initial 48-week double-blind phase of SELECT-COMPARE were subsequently eligible to cross over into the ongoing open-label extension study. At the current 156-week analysis, 32% of patients in the UPA + MTX arm and 22% in the ADA + MTX arm were in remission (P<0.001) [29].
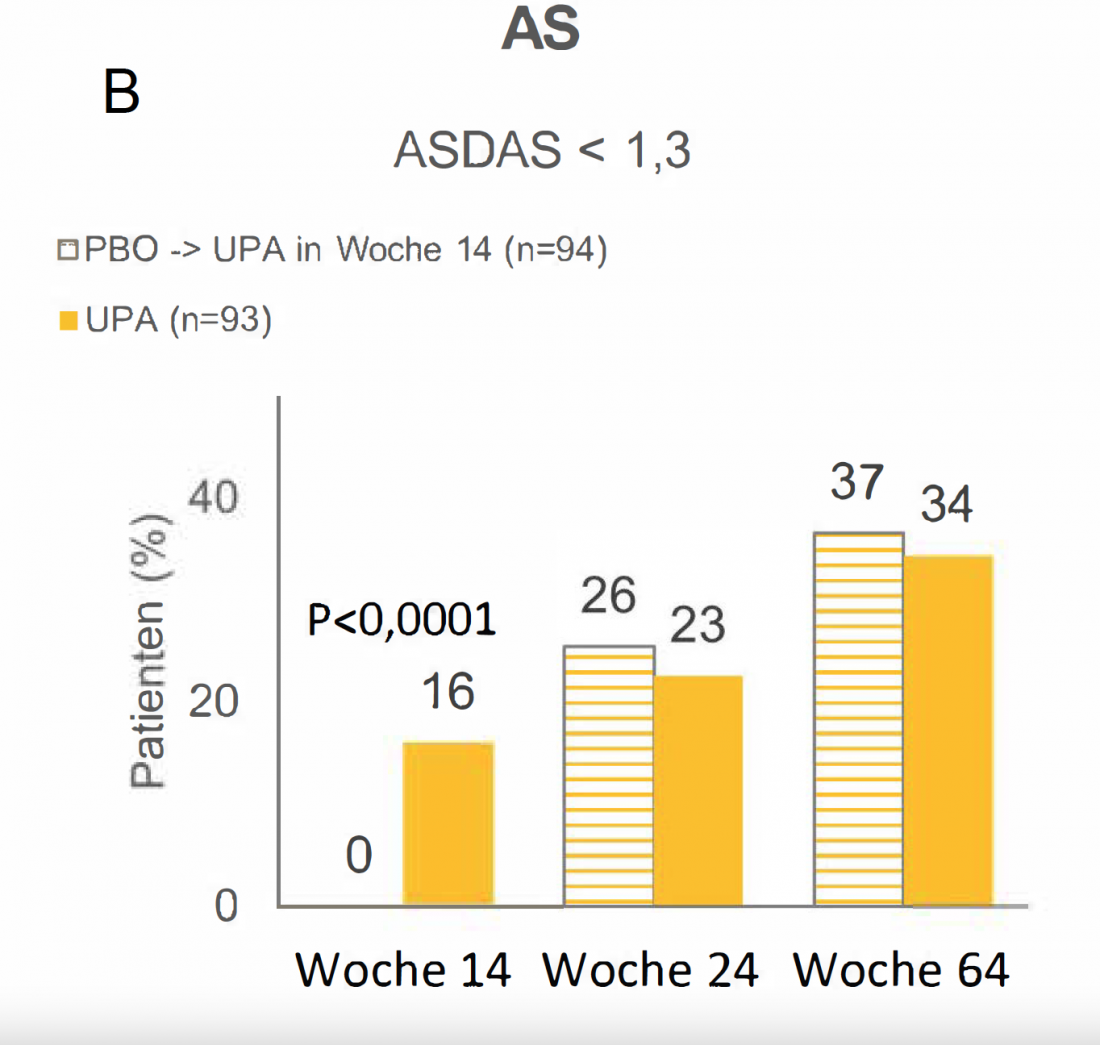
UPA in AS – Almost every 4th patient in remission after 24 weeks [30]
At the conclusion of the 14-week double-blind phase of the randomized phase IIb/III SELECT-AXIS 1 trial, 16% of AS patients on UPA who had previously responded inadequately to nonsteroidal anti-inflammatory drugs (NSAIDs) were in ASDAS < 1,3 remission. Under PBO, this was not the case for any patient (Fig. 1B) (P<0.0001) [27]. From week 14, all patients in the PBO arm received UPA. As early as 10 weeks after the switch, the remission rate of 26% was comparable to the remission rate of 23% among patients who had received UPA continuously for 24 weeks. After a total of 64 weeks, more than 34% of patients in both groups were in remission [30].
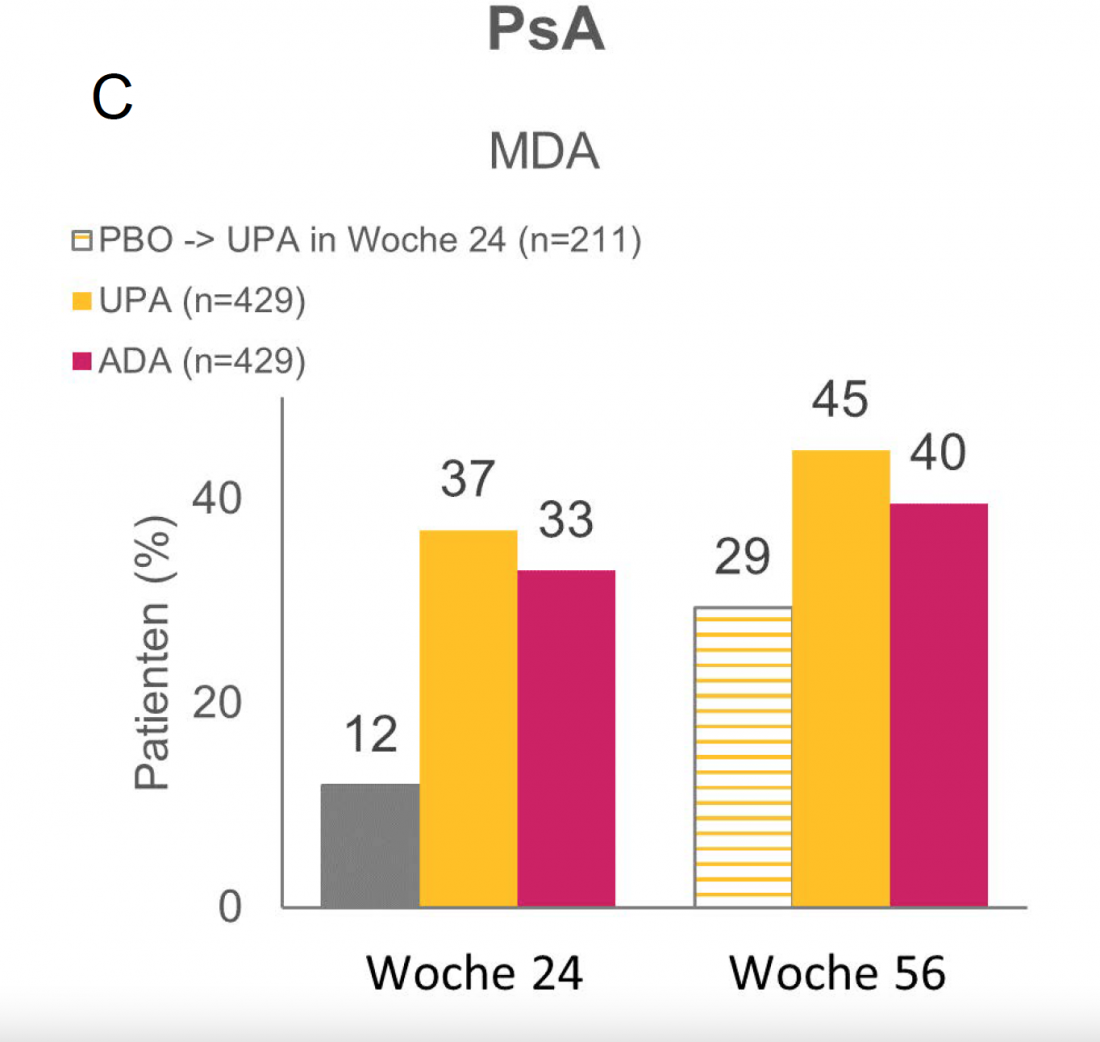
UPA in PsA – remission in more than 3 of 10 patients at week 24. [31]
Of PsA patients with inadequate response or intolerance to ≥ 1 conventional synthetic disease-modifying antirheumatic drug (csDMARD) treated with UPA in the randomized phase III SELECT-PsA 1 trial, 37% met criteria for MDA remission at 24 weeks, compared with 33% in the ADA arm and 12% in the PBO arm (Fig. 1C). Subsequently, all patients from the PBO group were switched to UPA. After a total of 56 weeks, 29% of these patients showed MDA remission. At this time, 45% of patients on continuous UPA treatment and 40% of patients on continuous ADA treatment had already achieved this [31].
Pain relief under UPA in all three indications already after 3 months [20, 25, 27].
Pain in the musculoskeletal system causes a high level of suffering for patients with RA, PsA and AS [1-3]. As the results of the various SELECT studies show, UPA can provide rapid, significant, and long-term relief of pain for sufferers. Thus, as assessed by RA patients in SELECT-COMPARE, pain had improved by 32.1 points on a scale of 0 to 100 after 12 weeks with UPA + MTX, compared with an improvement of 25.6 points with ADA + MTX and 15.7 points with PBO + MTX (P UPA vs. PBO and UPA vs. ADA each ≤0.001) [20]. Pain relief under UPA was maintained over 156 weeks (-39.8 points under UPA vs. -31.2 points under ADA; P<0.001) [29].
AS patients in SELECT-AXIS 1 experienced a significant reduction in back pain after only 2 weeks of continuous treatment with UPA. After 12 weeks, a scale of 0 to 10 showed an improvement of 3.22 points under UPA and 1.38 points in the PBO group (P<0.0001) [27]. After 64 weeks, back pain had further improved with an overall reduction of 4.95 points compared with baseline under UPA [30].
In the SELECT-PsA 1 trial, patients in the UPA and ADA arms each experienced a mean pain reduction of 2.3 points at 12 weeks and 0.9 points under PBO [25]. Again, after a longer observation period of 56 weeks, there was further improvement in pain (-3.3 points under UPA vs. -2.9 points under ADA) [31].
Stable and well-studied safety profile [30-32]
In addition to efficacy, long-term safety is a particularly important criterion in the selection of a suitable therapy for chronic diseases. The European Medicines Agency (EMA) has currently initiated an evaluation of safety in the treatment of chronic inflammatory diseases for all approved JAKi [33]. As shown in a recent integrated analysis of clinical trials of UPA in RA, the safety profile of JAKi was stable and similar to that of ADA over a 4.5-year observation period, with the exception of more frequent occurrence of herpes zoster and increased creatine phosphokinase levels with UPA. Venous thromboembolism (VTE), malignancy, serious cardiovascular events (MACE), or serious infections were comparably frequent under 1 × daily 15 mg UPA as under PBO and ADA [32]. Also, no new safety signals for UPA were recorded in the PsA and AS studies [30, 31].
Conclusion
Remission is the primary treatment goal in inflammatory rheumatic diseases [1, 3, 4]. UPA may help enable an increasing number of patients with RA, PsA, and AS to reach this state in which they are no longer aware of their disease [30, 31, 34]. Thus, significant pain relief was achieved with UPA in all 3 indications after only 3 months [20, 25, 27]. After 3 years, more than one-third of RA patients in SELECT-COMPARE were still in remission with UPA + MTX, and current data from the AS and PsA trials at 64 and 56 weeks, respectively, also indicate that the state of remission can be maintained long-term with UPA [29-31]. JAKi, which can be taken as a tablet, also offers a well-studied and stable safety profile and can be used as monotherapy without MTX [15, 30-32].
Questions to Prof. Axel Finckh, Université de Genève:

What does achieving remission mean for patients with inflammatory rheumatic diseases?
The greatest desire of sufferers is to be out of pain, and a low level of pain is the factor that best correlates with a high quality of life. This is something we should always remember as rheumatologists. The needs of the patient are well aligned with the concept of remission: If a patient is in remission, he or she may no longer suffer from arthralgias. Furthermore, in the remission state, it should be ensured that there is little or no structural joint damage and collateral damage, such as cardiovascular disease, is unlikely. While the exact criteria used to define remission differ between RA, PsA, and AS. However, the expectation of patients is similar for all diseases: to live a normal life as before.
How realistic is achieving remission these days?
Several studies have shown that setting a quantifiable treatment goal and reviewing it regularly (“tight control”) leads to better disease control than usual follow-up. Communication between doctor and patient also plays an important role here. This approach is currently proposed in the main international treatment recommendations. It is the responsibility of the physician to individually adjust the treatment goals. Remission is often difficult to achieve in patients with nociceptive pain, advanced disease, and structural joint damage. In this case, it may even be counterproductive to pursue this goal, as this involves numerous and often unnecessary therapy adjustments. About one-quarter to one-third of patients with inflammatory rheumatic diseases show a nociceptive component in their arthralgias, which the physician should identify and treat specifically.
What has been your experience with the use of UPA in your patients with RA, PsA, and AS?
I was pleasantly surprised at how quickly UPA took effect in several of my patients – even those who had previously responded inadequately to multiple biologic disease-modifying DMARDs. Rapid response is an important factor in patient acceptance of treatment. If there are not several months between “before” and “after,” they perceive the difference more clearly and are more likely to retain treatment long-term.
Over one year of RINVOQ® in AS and PsA!On March 23, 2021, UPA received Swissmedic approval for the indications AS and PsA [35]. Thus, patients with these inflammatory rheumatic diseases can benefit from targeted oral therapy for more than a year. For moderate to severe RA, UPA can be used as early as January 2020 [36]. Thus, UPA is the first and currently the only JAKi approved in RA, AS, and PsA [15, 37]. UPA is taken in tablet form with a recommended dosage of 15 mg 1x daily with or food [15]. The cost of treatment with UPA is covered by health insurance in all three indications [38].
|
Literature:
The references can be checked by specialists at medinfo.ch@abbvie.com can be requested.
Brief technical information RINVOQ®
Dr. sc. nat. Jennifer Keim
With the financial support of AbbVie AG, Alte Steinhauserstrasse 14, 6330 Cham.
Article online since 07.06.2022
CH-RNQR-220050_05/2022