Data from the current SEQUENCE study was presented at the United European Gastroenterology Week (UEGW), which took place from October 14 to 17, 2023, and has since been published [1,2]. In der randomisierten Phase-III-Head-to-Head-Studie wurde Risankizumab (SKYRIZI®) mit Ustekinumab zur Behandlung erwachsener Patient:innen mit mittelschwerem bis schwerem aktivem Morbus Crohn (CD, Crohn’s Disease) verglichen, bei denen eine oder mehrere Anti-TNF-Therapien versagt hatten [1]. Risankizumab achieved all primary and secondary endpoints compared to ustekinumab and demonstrated superiority [1]. SEQUENCE is therefore the only head-to-head study in Crohn’s disease to show superiority of a biologic over another biologic.
Crohn’s disease is extremely stressful for those affected: the underlying inflammation can cause permanent intestinal damage and significantly restrict the quality of life of those affected [3, 4]. In Switzerland, two approved interleukin (IL)-23 and IL-12/-23 inhibitors, risankizumab and ustekinumab, respectively, are available for the treatment of Crohn’s disease [5, 6]. Risankizumab has been approved in Switzerland since 14.09.2023 for the treatment of adult patients with moderate to severe active Crohn’s disease who have responded inadequately to conventional therapy or a biologic, have stopped responding or have not tolerated it [5]. As risankizumab leads to significant clinical and endoscopic remission and can therefore contribute to mucosal healing, it is an important milestone in the therapeutic landscape of Crohn’s disease [7, 8].
The SEQUENCE study in detail [1]
Over 500 patients with failure to respond to one or more TNF inhibitors were randomized to 48 weeks of open-label treatment with either risankizumab (N=255, 3x 600 mg i.v. in weeks 0, 4 and 8; 5x 360 mg s.c. in weeks 12, 20, 28, 36 and 44) or ustekinumab (N=265, 1x 260/390/520 mg i.v. in week 0; 5x 90 mg s.c. in weeks 8, 16, 24, 32 and 40) [1]. The first primary endpoint was clinical remission according to the Crohn’s Disease Activity Index (CDAI), defined as CDAI < 150 after 24 weeks, tested for non-inferiority. The second primary endpoint was endoscopic remission according to the Simple Endoscopic Score for Crohn’s Disease (SES-CD), defined as SES-CD ≤ 4 and at least 2 points lower than baseline at 48 weeks, tested for superiority. The endoscopic results were evaluated in a blinded central reading. Baseline demographics were balanced in both treatment arms [1].
Risankizumab met both primary endpoints [1]
By the 48-week readout point, 90.2% of risankizumab patients were still participating in the study compared to 72.8% of ustekinumab patients. One of the main reasons for early discontinuation in the ustekinumab arm was a lack of efficacy in 13.2% of patients, while only 2.0% of risankizumab patients withdrew from the study for this reason [1]. Both primary endpoints of the study were met (Fig. 1). After 24 weeks, 58.6 % of patients in the risankizumab group achieved clinical remission compared to 39.5 % of ustekinumab patients, demonstrating non-inferiority of risankizumab to ustekinumab with a non-inferiority margin of 10 % [1]. In addition, risankizumab already showed an indication of superiority over ustekinumab after 24 weeks (p < 0.01, post-hoc analysis for superiority) [2]. In the second co-primary endpoint, endoscopic remission, risankizumab was found to be superior to ustekinumab after 48 weeks. Almost twice as many patients receiving risankizumab achieved endoscopic remission compared to ustekinumab (31.8 % vs. 16.2 %, p < 0.0001) [1].
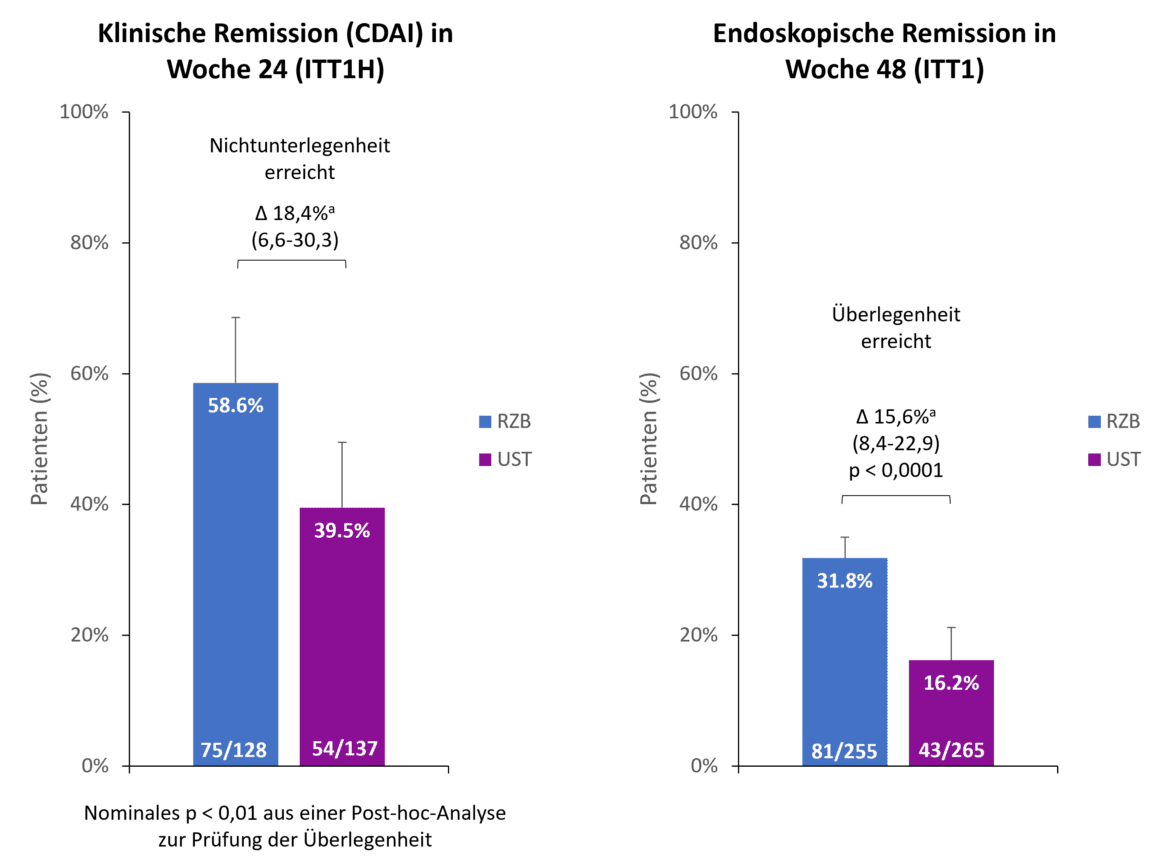
Fig. 1. Primary endpoints: Risankizumab (RZB) demonstrated non-inferiority to ustekinumab (UST) in achieving clinical remission at week 24 and superiority to UST in achieving endoscopic remission at week 48. a Differences are adjusted for stratification factors (number of unsuccessful prior anti-TNF therapies [≤ 1, > 1] and steroid use at baseline [yes, no]). ITT1H: randomized, treated patients who were treated for at least 24 weeks at the time of analysis (interim analysis at week 24); ITT1: randomized patients who received at least 1 dose of RZB resp. UST; CDAI: Crohn’s Disease Activity Index; RZB: Risankizumab; UST: Ustekinumab. Adapted from [1, 2].
Risankizumab showed superiority over ustekinumab in all secondary endpoints [1]
Risankizumab also showed superiority compared to ustekinumab in the secondary endpoints of the SEQUENCE study, clinical remission after 48 weeks, endoscopic response after 24 and 48 weeks and steroid-free endoscopic and clinical remission after 48 weeks (Fig. 2). For example, 60.8 % of risankizumab patients were in clinical remission after 48 weeks compared to 40.8 % of ustekinumab patients (p<0.0001). The difference in endoscopic response between risankizumab and ustekinumab was even more pronounced after 48 weeks than after 24 weeks (45.1 % vs. 21.9 %, p<0.0001) [1].
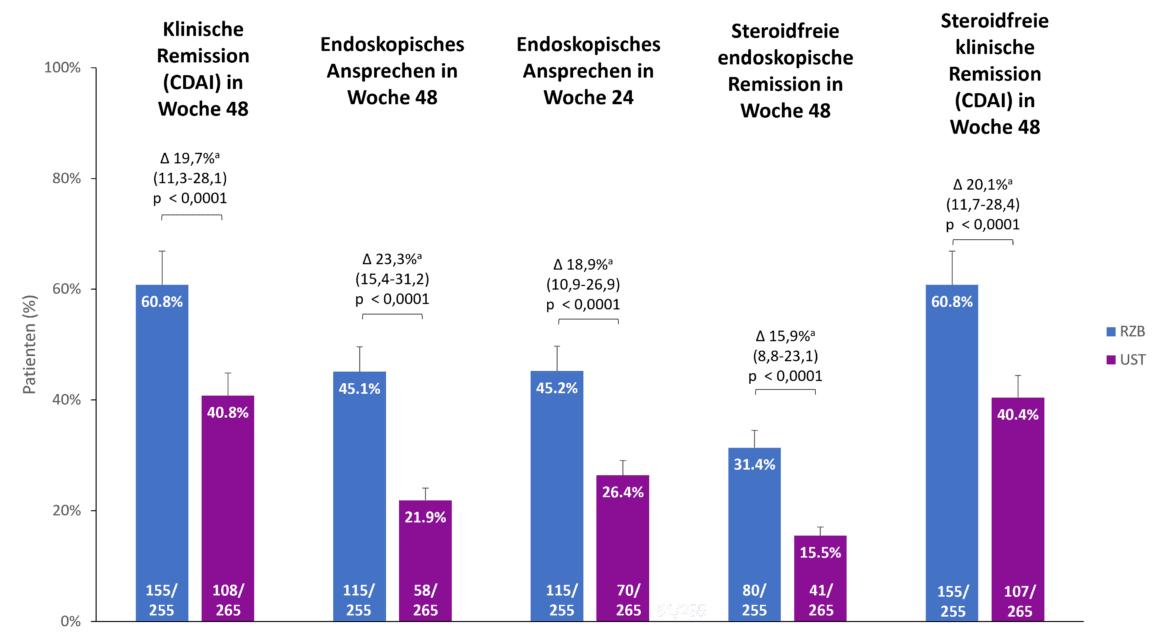
Fig. 2. Risankizumab (RZB) showed superiority over ustekinumab (UST) in all secondary endpoints. a The differences are adjusted using the stratification factors (number of unsuccessful previous anti-TNF therapies [≤ 1, > 1] and steroid use at the start of the study [yes, no]). CDAI: Crohn’s Disease Activity Index; RZB: Risankizumab; UST: Ustekinumab. Adapted from [1, 2].
Safety profile of risankizumab
The overall incidence of treatment-emergent adverse events (TEAEs) was low for risankizumab and ustekinumab (27.9% vs. 21.9%). 16.0% of patients in the risankizumab arm had serious adverse events compared to 19.2% in the ustekinumab arm. In both treatment arms, there were few TEAEs that led to discontinuation of the study drug (risankizumab 3.8% vs. ustekinumab 4.9%) [1]. Compared to the pivotal studies, no new safety signals were identified with risankizumab [1, 7, 8].
Conclusion
The latest data from the SEQUENCE study confirm the recently approved biologic risankizumab as an effective treatment option for patients with Crohn’s disease. In a head-to-head comparison with ustekinumab in anti-TNF-refractory patients, risankizumab proved superior and all primary and secondary endpoints were met [1]. Clinical as well as endoscopic remission and mucosal healing as therapeutic goals are thus coming closer and patients with Crohn’s disease can hope for lower disease activity and look forward to a promising new therapy.
Short technical information SKYRIZI®
Literature
- Peyrin-Biroulet, L., et al. Risankizumab versus ustekinumab for moderate-to-severe Crohn’s disease. N Engl J Med, 2024. 391(3): p. 213-223.
- Peyrin-Biroulet, L., et al. Risankizumab Versus Ustekinumab for Patients With Moderate to Severe Crohn’s Disease: Results From the Phase 3b SEQUENCE Study. UEGW; Copenhagen, Oct 14-16, 2023.
- Jairath, V. and B.G. Feagan, Global burden of inflammatory bowel disease. Lancet Gastroenterol Hepatol, 2020. 5(1): p. 2-3.
- The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol, 2020. 5(1): p. 17-30.
- Current expert information on SKYRIZI® (risankizumab) Crohn’s disease at www.swissmedicinfo.ch.
- Current technical information for ustekinumab at www.swissmedicinfo.ch.
- D’Haens, G., et al, Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet, 2022. 399(10340): p. 2015-2030.
- Ferrante, M., et al, Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomized, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial.Lancet, 2022. 399(10340): p. 2031-2046.
The references can be requested by specialists at medinfo.ch@abbvie.com.
Report: Dr. sc. nat. Stefanie Jovanovic
This article was produced with the financial support of AbbVie AG, Alte Steinhauserstrasse 14, Cham.
CH-SKZG-240059 09/2024
This article has been released in German.