In October, the annual congress of the German, Austrian and Swiss Societies of Hematology and Medical Oncology was held in Basel. A scientific symposium focused on risk- and age-adapted management in multiple myeloma. Myeloma patients over 75 years of age may benefit from adapted, less toxic treatment because of fewer side effects and less frequent need to discontinue therapy due to adverse effects.
The diagnosis and treatment of multiple myeloma (MM) has changed considerably in recent years. The assessment of the patient’s individual risk is becoming increasingly important. Therefore, Dr. med. Marc Raab, Heidelberg University Hospital (D), posed the question in his lecture: Which therapy is right: risk-adapted, MRD-targeted or still “one size fits all”?
Comorbidities influence the choice of therapeutic agents
Increasingly individualized treatment of MM is based on an understanding of how patient-, disease-, and treatment-specific factors affect survival rates and quality of life. Age has long been considered the most important and sole parameter for assessing transplantability. Today, however, it is known that age alone (up to about 75 years) is not a reason not to perform stem cell transplantation. Also important in assessing the patient’s ability to receive therapy are comorbidities, cytogenetics, and knowledge of drug-specific toxicities.
Renal insufficiency is a significant comorbidity and negatively associated with prognosis. 30% of all MM patients are affected in the course of the disease, in 10% even hemodialysis is necessary. The drug of choice in these patients is bortezomib.
Toxicities also have implications for drug choice; for example, caution is warranted in the use of anthracyclines or carfilzomib in patients with cardiac disease or agents that induce neuropathy in patients with diabetes mellitus.
In recent years, more has been learned about the genetic and clinical heterogeneity of MM disease. Current guidelines recognize the value of a risk-adapted approach based on FISH analysis, among other factors. Different MM subtypes respond differently to different drug classes. Patients with del(17p) and/or t(4;14) and/or +1q21 have a rather unfavorable prognosis. Patients with a t(4;14)-MM respond well to bortezomib, and in patients with a 17p deletion, conventional chemotherapy has limited effect.
What is the role of “minimal residual disease” (MRD)?
In first-line therapy, remission can be achieved in almost all patients. However, minimal residual disease (MRD) remains a problem. Different methods are available for their diagnosis, all of which have advantages and disadvantages. Flow cytometry is the best documented and fast (result is available the next day), genetic diagnosis takes longer. MRD has high prognostic significance; therefore, an important therapeutic goal is for as many patients as possible to become MRD-free. However, there are many unanswered questions regarding MRD:
- What technique should be used to diagnose it (reliability, availability)?
- When should the diagnosis be made?
- What are the consequences of which MRD status?
The speaker felt that as yet MRD assessment is not suitable for everyday clinical use, but it should be undertaken in clinical trials. These could answer a number of questions, such as whether maintenance therapy should be adjusted based on MRD or whether MRD could also play a role in the approval of new drugs.
Stem cell transplantation for MM relapse
At the beginning of his presentation, Prof. Nicolaus Kröger, MD, University Medical Center Hamburg-Eppendorf (D), defined salvage stem cell transplantation (STX): autologous or allogeneic STX in patients who have failed prior therapy. This includes patients who have had previous STX, but also patients without previous STX. Salvage STX have been increasingly performed in Europe since 2006. Conditioning is almost always done with melphalan.
Retrospective studies show that in autologous salvage STX, the following factors favorably influence outcome: longer remission after initial STX, younger age of the patient, low levels of beta2-microglobulin, and fewer salvage therapies before salvage STX.
In a prospective study by Cook et al. patients with MM relapse were treated either with autologous salvage STX or conventionally with cyclophosphamide [1]. Overall survival was the same in both groups, but time to progression (PFS) was significantly longer in the patient group with STX (19 vs. 11 months).
The current guidelines (2015) for salvage STX include the following principles [2]:
- In transplant-eligible patients with relapse after primary therapy that did not consist of autologous STX, autologous STX as part of salvage therapy should be considered standard.
- Autologous salvage STX is an appropriate therapy for patients with relapse in whom remission after initial autologous STX has lasted longer than 18 months.
- Autologous salvage STX can be used as bridging therapy to allogeneic STX.
Currently, early autologous salvage STX for recurrence is being studied in the prospective GMMG trial. Another area of research is strategies to address MRD after transplantation. There are different strategies for this, e.g. immunotherapy or vaccination. There are still few data comparing autologous with allogeneic salvage STX. These are more in favor of autologous STX, but these are retrospective data from selected patients. The speaker’s conclusion:
- Both autologous and allogeneic STX as salvage therapy in patients with MM relapse are effective treatment options and are increasingly used.
- Prolonged remission after the first STX is a positive prognostic factor for the second STX.
- Maintenance therapy after STX needs further investigation.
- Allogeneic STX has a lower risk of MM recurrence, but recurrence-independent mortality is higher than autologous STX.
- Currently, new therapeutic regimens with attenuated toxicity are being investigated.
Elderly patients with MM: determine frailty
MM is predominantly a disease of older people: More than 60% of diagnoses and about 75% of deaths as a result of MM affect people over 65 years of age, as PD Dr. med. Katja Christina Weisel, University Hospital Tübingen (D), explained in her lecture. In recent years, the prognosis in MM has improved significantly thanks to new therapeutic options. In a cohort of patients diagnosed from 2001-2005, the median survival was 4.6 years, and in the cohort diagnosed from 2006 to 2010, the median survival was already 6.1 years. Patients over 70 years of age benefit less from these advances than younger patients, but several studies have shown that the new agents significantly improve survival even in MM patients over 75 years of age. Toxicities are particularly important in this patient group, as grade 3-4 hematologic toxicities and treatment interruptions due to side effects reduce survival. In elderly patients, therapies should therefore be well tolerated.
However, elderly patients form a very heterogeneous group: some are still very fit, others frail. In frail individuals, resistance to stressors – such as cancer and its therapy – is diminished. But how should one assess a patient’s frailty? In a recently published study, Palumbo et al. presented an assessment tool that can calculate the likelihood of toxicity and survival in elderly patients with newly diagnosed MM, based on factors such as age, functional status, and comorbidities [3]. As of October 1, 2015, this calculation tool can be found online at www.myelomafrailtyscorecalcu lator.net.
Dual regimens in patients over 75
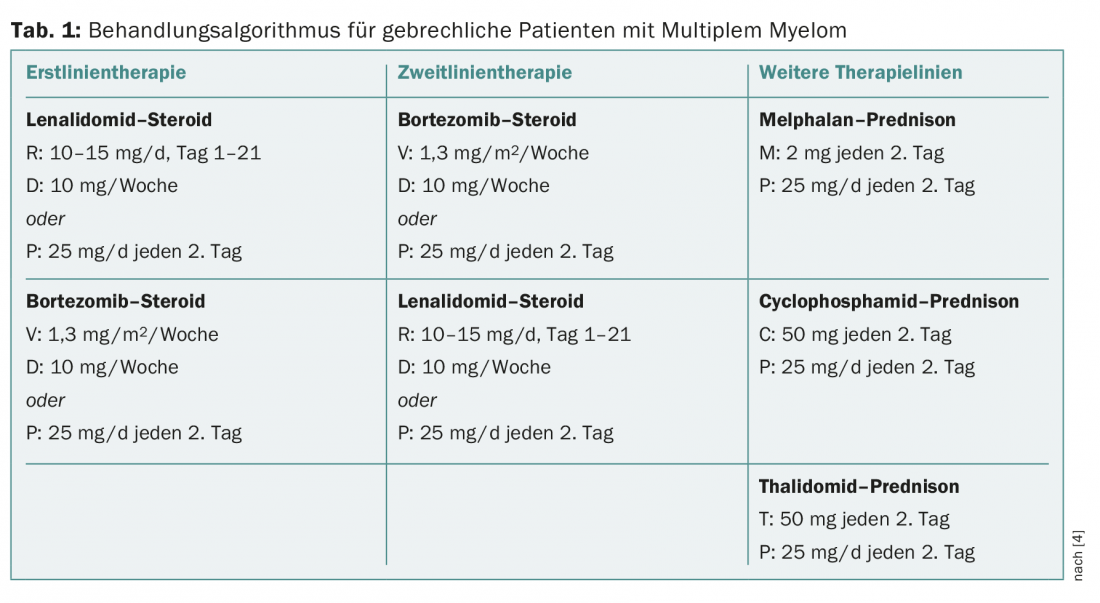
Standard therapy for newly diagnosed MM is usually a triple combination. However, in patients older than 75 years, these regimens cause a high rate of side effects. Recent studies show that a dual regimen in patients over 75 years of age reduces adverse event rates and improves survival. In frail patients, it is recommended to give bortezomib only once a week: The survival rate does not decrease, significantly fewer polyneuropathies occur, and therapy discontinuations occur less frequently. In the FIRST trial, it was shown that it is also possible to halve the dose of dexamethasone in patients older than 75 years without decreasing survival. The speaker made the following recommendations for the therapy of MM patients over 75 years of age (Table 1):
- Dose lenalidomide more carefully;
- Fully dose bortezomib, but administer only once weekly;
- Cut dexamethasone dose in half.
Source: Annual Meeting of the German, Austrian and Swiss Societies of Hematology and Medical Oncology, October 9-13, 2015, Basel.
Literature:
- Cook G, et al: High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label, phase 3 trial. Lancet Oncol 2014 Jul; 15(8): 874-885.
- Giralt S, et al: American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol Blood Marrow Transplant 2015 Sep 30. pii: S1083-8791(15)00641-2. doi: 10.1016/j.bbmt.2015.09.016.
- Palumbo A, et al: Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 2015; 125(13): 2068-2074.
- Larocca A, Palumbo A: How I treat fragile myeloma patients. Blood 2015 Aug 31. pii: blood-2015-05-612960.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(11-12): 28-31.